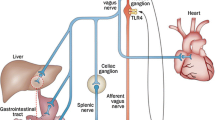
Abstract
Immune-mediated inflammatory diseases (IMIDs) are a group of common heterogeneous disorders, characterized by an alteration of cellular homeostasis. Primarily, it has been shown that the release and diffusion of neurotransmitters from nervous tissue could result in signaling through lymphocyte cell-surface receptors and the modulation of immune function. This finding led to the idea that the neurotransmitters could serve as immunomodulators. It is now manifested that neurotransmitters can also be released from leukocytes and act as autocrine or paracrine modulators. Increasing data indicate that there is a crosstalk between inflammation and alterations in neurotransmission. The primary goal of this review is to demonstrate how these two pathways may converge at the level of the neuron and glia to involve in IMID. We review the role of neurotransmitters in IMID. The different effects that these compounds exert on a variety of immune cells are also reviewed. Current and future developments in understanding the cross-talk between the immune and nervous systems will undoubtedly identify new ways for treating immune-mediated diseases utilizing agonists or antagonists of neurotransmitter receptors.
Similar content being viewed by others
References
Reale M, Conti L, Velluto D (2018) Immune and inflammatory-mediated disorders: from bench to bedside. J Immunol Res 2018
Bellinger DL, Lorton D (2018) Sympathetic nerve hyperactivity in the spleen: causal for nonpathogenic-driven chronic immune-mediated inflammatory diseases (IMIDs)? Int J Mol Sci 19(4):1188
García M et al (2020) Impact of immune-mediated diseases in inflammatory bowel disease and implications in therapeutic approach. Sci Rep 10(1):1–9
Procaccini C et al (2014) Neuro-endocrine networks controlling immune system in health and disease. Front Immunol 5:143
Dhaiban S et al (2021) Role of peripheral immune cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Sci 3(1):12
Capellino S (2020) Dopaminergic agents in rheumatoid arthritis. J Neuroimmune Pharmacol 15(1):48–56
Halling ML et al (2017) Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol 23(33):6137
Khalil M, Zhang Z, Engel MA (2019) Neuro-immune networks in gastrointestinal disorders. Visc Med 1(1):52–60
Ananthakrishnan AN et al (2018) Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol 15(1):39–49
Burgaletto C et al (2020) The immune system on the TRAIL of Alzheimer’s disease. J Neuroinflammation 17(1):1–11
Pajares M et al (2020) Inflammation in Parkinson’s disease: mechanisms and therapeutic implications. Cells 9(7):1687
Klein JR (2021) Dynamic interactions between the immune system and the neuroendocrine system in health and disease. Front Endocrinol 12:278
Webster JI, Tonelli L, Sternberg EM (2002) Neuroendocrine regulation of immunity. Annu Rev Immunol 20(1):125–163
Tsoli M, Boutzios G, Kaltsas G (2019) Immune system effects on the endocrine system, in Endotext [Internet]. MDText.com, Inc.
Terrando N, Pavlov VA (2018) Neuro-immune interactions in inflammation and autoimmunity. Front Immunol 9:772
Pongratz G, Straub RH (2014) The sympathetic nervous response in inflammation. Arthritis Res Ther 16(6):504
Kerage D et al (2019) Interaction of neurotransmitters and neurochemicals with lymphocytes. J Neuroimmunol 332:99–111
Pacheco R, Riquelme E, Kalergis AM (2010) Emerging evidence for the role of neurotransmitters in the modulation of T cell responses to cognate ligands. Cent Nerv Syst Agents Med Chem (Formerly Current Medicinal Chemistry-Central Nervous System Agents) 10(1):65–83
Dantzer R (2018) Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev 98(1):477–504
Hodo TW et al (2020) Critical neurotransmitters in the neuroimmune network. Front Immunol 11:1869
Pavlov VA, Tracey KJ (2015) Neural circuitry and immunity. Immunol Res 63(1–3):38–57
Huston JM (2012) The vagus nerve and the inflammatory reflex: wandering on a new treatment paradigm for systemic inflammation and sepsis. Surg Infect 13(4):187–193
Taub DD (2008) Neuroendocrine interactions in the immune system. Cell Immunol 252(1–2):1
Deckx N, Lee WP, Berneman ZN, Cools N (2013) Neuroendocrine immunoregulation in multiple sclerosis. Clin Dev Immunol 2013:705232. https://doi.org/10.1155/2013/705232. Epub 2013 Dec 8
Chen L et al (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9(6):7204
Müller N (2018) Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull 44(5):973–982
Sam C, Bordoni B (2022) Physiology, Acetylcholine. In: StatPearls [Internet]. StatPearls Publishing
Tiwari P et al (2013) Basic and modern concepts on cholinergic receptor: a review. Asian Pacific J Trop Dis 3(5):413–420
Hoover DB (2017) Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol Ther 179:1–16
Fujii T et al (2017) Expression and function of the cholinergic system in immune cells. Front Immunol 8:1085
Bosmans G et al (2017) Cholinergic modulation of type 2 immune responses. Front Immunol 8:1873
Báez-Pagán CA, Delgado-Vélez M, Lasalde-Dominicci JA (2015) Activation of the macrophage α7 nicotinic acetylcholine receptor and control of inflammation. J Neuroimmune Pharmacol 10(3):468–476
Zoli M et al (2018) Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr Neuropharmacol 16(4):338–349
McAllen RM et al (2015) The interface between cholinergic pathways and the immune system and its relevance to arthritis. Arthritis Res Ther 17(1):1–9
Kaushik V et al (2018) Acetylcholinesterase inhibitors: beneficial effects on comorbidities in patients with Alzheimer’s disease. Am J Alzheimers Dis Other Demen® 33(2):73–85
Di Bari M et al (2016) Dysregulated homeostasis of acetylcholine levels in immune cells of RR-multiple sclerosis patients. Int J Mol Sci 17(12):2009
Zabrodskii P (2011) Effect of acetylcholine on mortality of mice from sepsis and proinflammatory cytokine production. Bull Exp Biol Med 150(3):340
Mizrachi T et al (2021) Suppression of neuroinflammation by an allosteric agonist and positive allosteric modulator of the α7 nicotinic acetylcholine receptor GAT107. J Neuroinflammation 18(1):1–14
Nicoletti CG et al (2019) Treatment with dimethyl fumarate enhances cholinergic transmission in multiple sclerosis. CNS Drugs 33(11):1133–1139
Kooi E-J et al (2011) Cholinergic imbalance in the multiple sclerosis hippocampus. Acta Neuropathol 122(3):313–322
Di Bari M, Pinto GD, Reale M, Mengod G, Tata AM (2017) Cholinergic system and neuroinflammation: implication in multiple sclerosis. Cent Nerv Syst Agents Med Chem 17(2):109–115. https://doi.org/10.2174/1871524916666160822115133
Miceli P, Jacobson K (2003) Cholinergic pathways modulate experimental dinitrobenzene sulfonic acid colitis in rats. Auton Neurosci 105(1):16–24
Hayashi S et al (2014) Nicotine suppresses acute colitis and colonic tumorigenesis associated with chronic colitis in mice. Am J Physiol-Gastrointest Liver Physiol 307(10):G968–G978
Ghia JE et al (2006) The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology 131(4):1122–1130
Munyaka P et al (2014) Central muscarinic cholinergic activation alters interaction between splenic dendritic cell and CD4+ CD25-T cells in experimental colitis. PLoS One 9(10):e109272
Ji H et al (2014) Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol 7(2):335–347
Pai YC, Yu LCH (2020) Is “Cholinergic” stimulus useful for ulcerative colitis treatment? Dig Dis Sci 65(1):6–8. https://doi.org/10.1007/s10620-019-05933-8
Bai A, Guo Y, Lu N (2007) The effect of the cholinergic anti-inflammatory pathway on experimental colitis. Scand J Immunol 66(5):538–545
Al-Khotani A, Alstergren P (2017) Acetylcholine suppresses release of interleukin-6 in fibroblast-like synov-iocytes in rheumatoid arthritis. J Dent Oro Surg 2(1):126
Yabut JM et al (2019) Emerging roles for serotonin in regulating metabolism: new implications for an ancient molecule. Endocr Rev 40(4):1092–1107
Fitzpatrick PF (2003) Mechanism of aromatic amino acid hydroxylation. Biochemistry 42(48):14083–14091
Berger M, Gray JA, Roth BL (2009) The expanded biology of serotonin. Annu Rev Med 60:355–366
Gershon MD (2013) 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 20(1):14
Ahern GP (2011) 5-HT and the immune system. Curr Opin Pharmacol 11(1):29–33
Herr N, Bode C, Duerschmied D (2017) The effects of serotonin in immune cells. Front Cardiovasc Med 4:48
Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velázquez MA, Garcés-Alvarez ME, Hurtado-Alvarado G, Quintero-Fabian S, Pavón L (2015) Immunomodulatory effects mediated by serotonin. J Immunol Res 2015:354957. https://doi.org/10.1155/2015/354957. Epub 2015 Apr 19
Nowak EC et al (2012) Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J Exp Med 209(11):2127–2135
Chabbi-Achengli Y et al (2016) Serotonin is involved in autoimmune arthritis through Th17 immunity and bone resorption. Am J Pathol 186(4):927–937
Bernardes M et al (2017) Serum serotonin levels and bone in rheumatoid arthritis patients. Rheumatol Int 37(11):1891–1898
Coates M et al (2017) The many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease. Aliment Pharmacol Ther 46(6):569–580
Shajib MS et al (2019) Characterization of serotonin signaling components in patients with inflammatory bowel disease. J Can Assoc Gastroenterol 2(3):132–140
Li N et al (2011) Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol 178(2):662–671
Motavallian A et al (2013) Involvement of 5HT3 receptors in anti-inflammatory effects of tropisetron on experimental TNBS-induced colitis in rat. BioImpacts: BI 3(4):169
Sittipo P et al (2022) The function of gut microbiota in immune-related neurological disorders: a review. J Neuroinflammation 19(1):1–17
Kwon YH et al (2019) Modulation of gut microbiota composition by serotonin signaling influences intestinal immune response and susceptibility to colitis. Cell Mol Gastroenterol Hepatol 7(4):709–728
Sochocka M et al (2019) The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—a critical review. Mol Neurobiol 56(3):1841–1851
Pérez LP, González RS, Lázaro EB (2015) Treatment of mood disorders in multiple sclerosis. Curr Treat Options Neurol 17(1):1–11
Stamoula E et al (2021) Antidepressants on multiple sclerosis: a review of in vitro and in vivo models. Front Immunol 12:677879
San Hernandez AM, Singh C, Valero DJ, Nisar J, Ramirez JIT, Kothari KK, Isola S, Gordon DK (2020) Multiple sclerosis and serotonin: potential therapeutic applications. Cureus 12(11):e11293
Aboukhatwa M, Dosanjh L, Luo Y (2010) Antidepressants are a rational complementary therapy for the treatment of Alzheimer’s disease. Mol Neurodegener 5(1):1–17
Hall BJ, Hamlin PJ, Gracie DJ, Ford AC (2018) The effect of antidepressants on the course of inflammatory bowel disease. Can J Gastroenterol Hepatol 2018:2047242. https://doi.org/10.1155/2018/2047242
Matt S, Gaskill P (2019) Where is dopamine and how do immune cells see it?: dopamine-mediated immune cell function in health and disease. J Neuroimmune Pharmacol 15(1):114–164. https://doi.org/10.1007/s11481-019-09851-4
Levite M (2012) Dopamine in the immune system: dopamine receptors in immune cells, potent effects, endogenous production and involvement in immune and neuropsychiatric diseases. In: Nerve-Driven Immunity. Springer, pp 1–45
Arreola R, Alvarez-Herrera S, Pérez-Sánchez G, Becerril-Villanueva E, Cruz-Fuentes C, Flores-Gutierrez EO, Garcés-Alvarez ME, de la Cruz-Aguilera DL, Medina-Rivero E, Hurtado-Alvarado G, Quintero-Fabián S, Pavón L (2016) Immunomodulatory effects mediated by dopamine. J Immunol Res 2016:3160486. https://doi.org/10.1155/2016/3160486. Epub 2016 Oct 4
Gurevich EV, Gainetdinov RR, Gurevich VV (2016) G protein-coupled receptor kinases as regulators of dopamine receptor functions. Pharmacol Res 111:1–16
Wang X et al (2019) The prospective value of dopamine receptors on bio-behavior of tumor. J Cancer 10(7):1622
Mackie P et al (2018) The dopamine transporter: an unrecognized nexus for dysfunctional peripheral immunity and signaling in Parkinson’s Disease. Brain Behav Immun 70:21–35
Vidal PM, Pacheco R (2019) Targeting the dopaminergic system in autoimmunity. J Neuroimmune Pharmacol 15(1):57–73. https://doi.org/10.1007/s11481-019-09834-5
Hoeger S et al (2008) Donor dopamine treatment in brain dead rats is associated with an improvement in renal function early after transplantation and a reduction in renal inflammation. Transpl Int 21(11):1072–1080
Beck GC et al (2001) Modulation of chemokine production in lung microvascular endothelial cells by dopamine is mediated via an oxidative mechanism. Am J Respir Cell Mol Biol 25(5):636–643
Kapper S et al (2002) Modulation of chemokine production and expression of adhesion molecules in renal tubular epithelial and endothelial cells by catecholamines. Transplantation 74(2):253–260
Capellino S et al (2014) Increased expression of dopamine receptors in synovial fibroblasts from patients with rheumatoid arthritis: inhibitory effects of dopamine on interleukin-8 and interleukin-6. Arthritis Rheumatol 66(10):2685–2693
Capellino S (2019) Dopaminergic agents in rheumatoid arthritis. J Neuroimmune Pharmacol 15(1):48–56
Nakano K et al (2011) Dopamine induces IL-6–dependent IL-17 production via D1-like receptor on CD4 naive T cells and D1-like receptor antagonist SCH-23390 inhibits cartilage destruction in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol 186(6):3745–3752
van Nie L et al (2020) Dopamine induces in vitro migration of synovial fibroblast from patients with rheumatoid arthritis. Sci Rep 10(1):1–13
Wieber K et al (2022) Dopamine receptor 1 expressing B cells exert a proinflammatory role in female patients with rheumatoid arthritis. Sci Rep 12(1):1–15
Magro F et al (2002) Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci 47(1):216–224
Magro F et al (2004) Decreased availability of intestinal dopamine in transmural colitis may relate to inhibitory effects of interferon-γ upon L-DOPA uptake. Acta Physiol Scand 180(4):379–386
Magro F et al (2006) Dopamine D 2 receptor polymorphisms in inflammatory bowel disease and the refractory response to treatment. Dig Dis Sci 51(11):2039–2044
Cosentino M et al (2007) Human CD4+ CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood 109(2):632–642
Pacheco R, Contreras F, Zouali M (2014) The dopaminergic system in autoimmune diseases. Front Immunol 5:117
Cosentino M, Zaffaroni M, Marino F (2014) Levels of mRNA for dopaminergic receptor D5 in circulating lymphocytes may be associated with subsequent response to interferon-β in patients with multiple sclerosis. J Neuroimmunol 277(1–2):193–196
Levite M, Marino F, Cosentino M (2017) Dopamine, T cells and multiple sclerosis (MS). J Neural Transm 124(5):525–542
Giorelli M, Livrea P, Trojano M (2005) Dopamine fails to regulate activation of peripheral blood lymphocytes from multiple sclerosis patients: effects of IFN-β. J Interferon Cytokine Res 25(7):395–406
Lieberknecht V et al (2017) Pramipexole, a dopamine D2/D3 receptor-preferring agonist, prevents experimental autoimmune encephalomyelitis development in mice. Mol Neurobiol 54(2):1033–1045
Zhu Y et al (2020) 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine Induced Parkinson’s disease in mouse: potential association between neurotransmitter disturbance and gut microbiota dysbiosis. ACS Chem Neurosci 11(20):3366–3376
Fung TC (2020) The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis 136:104714
Zhang T et al (2022) Gut microbiota relieves inflammation in the substantia nigra of chronic Parkinson’s disease by protecting the function of dopamine neurons. Exp Ther Med 23(1):1–10
Hamamah S et al (2022) Role of microbiota-gut-brain axis in regulating dopaminergic signaling. Biomedicines 10(2):436
Everington EA et al (2018) Molecular characterization of GABA-A receptor subunit diversity within major peripheral organs and their plasticity in response to early life psychosocial stress. Front Mol Neurosci 11:18
Sigel E, Steinmann ME (2012) Structure, function, and modulation of GABAA receptors. J Biol Chem 287(48):40224–40231
Jin Z, Mendu SK, Birnir B (2013) GABA is an effective immunomodulatory molecule. Amino Acids 45(1):87–94
Wu C et al (2017) The immunological function of GABAergic system. Front Biosci (Landmark edition) 22:1162
Auteri M, Zizzo MG, Serio R (2015) GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res 93:11–21
Han D et al (2007) Wound healing activity of gamma-aminobutyric Acid (GABA) in rats. J Microbiol Biotechnol 17(10):1661–1669
Reyes-García MG et al (2007) GABA (A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J Neuroimmunol 188(1–2):64–68
Tian J et al (1999) GABAA receptors mediate inhibition of T cell responses. J Neuroimmunol 96(1):21–28
Song D-K et al (1998) Central GABAA and GABAB receptor modulation of basal and stress-induced plasma interleukin-6 levels in mice. J Pharmacol Exp Ther 287(1):144–149
Sanders RD et al (2013) Benzodiazepine augmented γ-amino-butyric acid signaling increases mortality from pneumonia in mice. Crit Care Med 41(7):1627
Alam S et al (2006) Human peripheral blood mononuclear cells express GABAA receptor subunits. Mol Immunol 43(9):1432–1442
Tian J et al (2004) γ-Aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol 173(8):5298–5304
Demakova E, Korobov V, Lemkina L (2003) Determination of gamma-aminobutyric acid concentration and activity of glutamate decarboxylase in blood serum of patients with multiple sclerosis. Klin Lab Diagn 4:15
Bhat R et al (2010) Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci 107(6):2580–2585
Ma X et al (2018) Activation of GABAA receptors in colon epithelium exacerbates acute colitis. Front Immunol 9:987
Tian J et al (2011) Oral GABA treatment downregulates inflammatory responses in a mouse model of rheumatoid arthritis. Autoimmunity 44(6):465–470
Kelley JM, Hughes LB, Bridges SL (2008) Does gamma-aminobutyric acid (GABA) influence the development of chronic inflammation in rheumatoid arthritis? J Neuroinflammation 5(1):1–5
Wiatrak B et al (2022) The role of the microbiota-gut-brain axis in the development of Alzheimer’s disease. Int J Mol Sci 23(9):4862
Platt SR (2007) The role of glutamate in central nervous system health and disease–a review. Vet J 173(2):278–286
Haroon E, Miller AH, Sanacora G (2017) Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology 42(1):193–215
McCullumsmith RE, Sanacora G (2015) Regulation of extrasynaptic glutamate levels as a pathophysiological mechanism in disorders of motivation and addiction. Neuropsychopharmacology 40(1):254
Wang J et al (2020) Molecular mechanisms of glutamate toxicity in Parkinson’s disease. Front Neurosci 14:1201
Hardingham GE, Bading H (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11(10):682–696
Malarkey EB, Parpura V (2008) Mechanisms of glutamate release from astrocytes. Neurochem Int 52(1–2):142–154
Duman RS (2014) Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci 16(1):11
McEwen BS, Nasca C, Gray JD (2016) Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41(1):3–23
Allam SL et al (2015) Synaptic efficacy as a function of ionotropic receptor distribution: a computational study. PLoS One 10(10):e0140333
Allam SL et al (2012) A computational model to investigate astrocytic glutamate uptake influence on synaptic transmission and neuronal spiking. Front Comput Neurosci 6:70
Hansen AM, Caspi RR (2010) Glutamate joins the ranks of immunomodulators. Nat Med 16(8):856–858
East SP, Gerlach K (2010) mGluR4 positive allosteric modulators with potential for the treatment of Parkinson’s disease: WO09010455. Expert Opin Ther Pat 20(3):441–445
Johnson KA, Conn PJ, Niswender CM (2009) Glutamate receptors as therapeutic targets for Parkinson’s disease. CNS Neurol Disord-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 8(6):475–491
Frigo M et al (2012) Glutamate and multiple sclerosis. Curr Med Chem 19(9):1295–1299
Chang C-H, Lin C-H, Lane H-Y (2020) d-glutamate and gut microbiota in Alzheimer’s disease. Int J Mol Sci 21(8):2676
O’Neill E, Harkin A (2018) Targeting the noradrenergic system for anti-inflammatory and neuroprotective effects: implications for Parkinson’s disease. Neural Regen Res 13(8):1332
Jiang L et al (2015) A novel role of microglial NADPH oxidase in mediating extra-synaptic function of norepinephrine in regulating brain immune homeostasis. Glia 63(6):1057–1072
Russo CD et al (2004) Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1β production. J Neuroinflammation 1(1):1–15
Culmsee C, Semkova I, Krieglstein J (1999) NGF mediates the neuroprotective effect of the β2-adrenoceptor agonist clenbuterol in vitro and in vivo: evidence from an NGF-antisense study. Neurochem Int 35(1):47–57
Simonini MV et al (2010) Increasing CNS noradrenaline reduces EAE severity. J Neuroimmune Pharmacol 5(2):252–259
Vollmar P et al (2009) The antidepressant venlafaxine ameliorates murine experimental autoimmune encephalomyelitis by suppression of pro-inflammatory cytokines. Int J Neuropsychopharmacol 12(4):525–536
Benarroch EE (2009) The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology 73(20):1699–1704
Samuels E, Szabadi E (2008) Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol 6(3):254–285
Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10(3):211–223
Marien MR, Colpaert FC, Rosenquist AC (2004) Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Rev 45(1):38–78
Polak PE, Kalinin S, Feinstein DL (2011) Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain 134(3):665–677
Chalermpalanupap T et al (2013) Targeting norepinephrine in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res Ther 5(2):1–9
Heneka M et al (2002) Noradrenergic depletion of the cortex potentiates amyloid beta-induced inflammation: implications for Alzheimer’s disease. Neurobiol Aging. Elsevier Science Inc 360 Park Ave South, New York 10010–1710
Heneka MT et al (2003) Noradrenergic depletion increases inflammatory responses in brain: effects on IκB and HSP70 expression. J Neurochem 85(2):387–398
Butkovich LM, Houser MC, Tansey MG (2018) α-synuclein and noradrenergic modulation of immune cells in Parkinson’s disease pathogenesis. Front Neurosci 12:626
Johnson M, Young AD, Marriott I (2017) The therapeutic potential of targeting substance P/NK-1R interactions in inflammatory CNS disorders. Front Cell Neurosci 10:296
Krause JE, Takeda Y, Hershey AD (1992) Structure, functions, and mechanisms of substance P receptor action. J Investig Dermatol 98(6):S2-7
Severini C et al (2002) The tachykinin peptide family. Pharmacol Rev 54(2):285–322
Zhang Z et al (2017) Up-regulated expression of substance P in CD8+ T cells and NK1R on monocytes of atopic dermatitis. J Transl Med 15(1):93
Hafidi A et al (2002) Comparative distribution of NK1, NK2, and NK3 receptors in the rat brainstem auditory nuclei. Brain Res 947(2):299–306
Harrison TA, Hoover DB, King MS (2004) Distinct regional distributions of NK1 and NK3 neurokinin receptor immunoreactivity in rat brainstem gustatory centers. Brain Res Bull 63(1):7–17
Todd AJ, McGill MM, Shehab SA (2000) Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci 12(2):689–700
Feistritzer C et al (2003) Natural killer cell functions mediated by the neuropeptide substance P. Regul Pept 116(1–3):119–126
Payan DG, Brewster D, Goetzl EJ (1983) Specific stimulation of human T lymphocytes by substance P. J Immunol 131(4):1613–1615
van der Kleij HP et al (2003) Functional expression of neurokinin 1 receptors on mast cells induced by IL-4 and stem cell factor. J Immunol 171(4):2074–2079
Germonpre P et al (1999) Presence of substance P and neurokinin 1 receptors in human sputum macrophages and U-937 cells. Eur Respir J 14(4):776–782
Chauhan VS et al (2008) Neurogenic exacerbation of microglial and astrocyte responses to Neisseria meningitidis and Borrelia burgdorferi. J Immunol 180(12):8241–8249
Marriott I, Bost KL (2001) Expression of authentic substance P receptors in murine and human dendritic cells. J Neuroimmunol 114(1–2):131–141
Levite M (2008) Neurotransmitters activate T-cells and elicit crucial functions via neurotransmitter receptors. Curr Opin Pharmacol 8(4):460–471
Martinez AN, Philipp MT (2016) Substance P and antagonists of the neurokinin-1 receptor in neuroinflammation associated with infectious and neurodegenerative diseases of the central nervous system. J Neurol Neuromed 1(2):29
Nessler S et al (2006) Suppression of autoimmune encephalomyelitis by a neurokinin-1 receptor antagonist—a putative role for substance P in CNS inflammation. J Neuroimmunol 179(1–2):1–8
Kang HS et al (2004) Neurokinin receptors: relevance to the emerging immune system. Arch Immunol Ther Exp-Engl Ed 52(5):338–347
Mashaghi A et al (2016) Neuropeptide substance P and the immune response. Cell Mol Life Sci 73(22):4249–4264
Ziebell JM, Morganti-Kossmann MC (2010) Involvement of pro-and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7(1):22–30
Ho W-Z et al (1997) Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol 159(11):5654–5660
Monastyrskaya K et al (2005) The NK1 receptor localizes to the plasma membrane microdomains, and its activation is dependent on lipid raft integrity. J Biol Chem 280(8):7135–7146
Hickey WF (1999) Leukocyte traffic in the central nervous system: the participants and their roles. In: Seminars in immunology, vol 11, no 2. Academic Press, pp 125–137
Whitney NP et al (2009) Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem 108(6):1343–1359
Lu H-C, Mackie K (2016) An introduction to the endogenous cannabinoid system. Biol Psychiat 79(7):516–525
Cabral GA, Ferreira GA, Jamerson MJ (2015) Endocannabinoids and the immune system in health and disease. In: Endocannabinoids. Springer, pp 185–211
Haugh O et al (2016) The emerging role of the cannabinoid receptor family in peripheral and neuro-immune interactions. Curr Drug Targets 17(16):1834–1840
Thompson Z et al (2017) Circulating levels of endocannabinoids respond acutely to voluntary exercise, are altered in mice selectively bred for high voluntary wheel running, and differ between the sexes. Physiol Behav 170:141–150
Patti F et al (2016) Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity. J Neurol Neurosurg Psychiatry 87(9):944–951
Sido JM, Nagarkatti PS, Nagarkatti M (2016) Production of endocannabinoids by activated T cells and B cells modulates inflammation associated with delayed-type hypersensitivity. Eur J Immunol 46(6):1472–1479
Chen D-J et al (2017) Brain cannabinoid receptor 2: expression, function and modulation. Acta Pharmacol Sin 38(3):312–316
Malfitano AM, Basu S, Maresz K, Bifulco M, Dittel BN (2014) What we know and do not know about the cannabinoid receptor 2 (CB2). Semin Immunol 26(5):369–79. https://doi.org/10.1016/j.smim.2014.04.002
Dittel B (2008) Direct suppression of autoreactive lymphocytes in the central nervous system via the CB2 receptor. Br J Pharmacol 153(2):271–276
Cencioni MT et al (2010) Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB 2 receptors. PLoS One 5(1):e8688
Gentili M et al (2019) Selective CB2 inverse agonist JTE907 drives T cell differentiation towards a Treg cell phenotype and ameliorates inflammation in a mouse model of inflammatory bowel disease. Pharmacol Res 141:21–31
Pacifici R et al (2003) Modulation of the immune system in cannabis users. JAMA 289(15):1929–1931
Fraguas-Sánchez AI, Torres-Suárez AI (2018) Medical use of cannabinoids. Drugs 78(16):1665–1703
Oláh A, Szekanecz Z, Bíró T (2017) Targeting cannabinoid signaling in the immune system:“High”-ly exciting questions, possibilities, and challenges. Front Immunol 8:1487
Katchan V, David P, Shoenfeld Y (2016) Cannabinoids and autoimmune diseases: a systematic review. Autoimmun Rev 15(6):513–528
Katz-Talmor D et al (2018) Cannabinoids for the treatment of rheumatic diseases—where do we stand? Nat Rev Rheumatol 14(8):488–498
Lehmann C et al (2016) Experimental cannabidiol treatment reduces early pancreatic inflammation in type 1 diabetes. Clin Hemorheol Microcirc 64(4):655–662
Hryhorowicz S, Kaczmarek-Ryś M, Zielińska A, Scott RJ, Słomski R, Pławski A (2021) Endocannabinoid system as a promising therapeutic target in inflammatory bowel disease–a systematic review. Front Immunol 12:790803. https://doi.org/10.3389/fimmu.2021.790803
Gado F et al (2018) Traditional uses of cannabinoids and new perspectives in the treatment of multiple sclerosis. Medicines 5(3):91
Zajicek JP et al (2005) Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry 76(12):1664–1669
Reynoso-Moreno I et al (2021) Selective endocannabinoid reuptake inhibitor WOBE437 reduces disease progression in a mouse model of multiple sclerosis. ACS Pharmacol Transl Sci 4(2):765–779
Corder G, Castro DC, Bruchas MR, Scherrer G (2018) Endogenous and exogenous opioids in pain. Annu Rev Neurosci 41:453–473. https://doi.org/10.1146/annurev-neuro-080317-061522
Henry MS, Gendron L, Tremblay ME, Drolet G (2017) Enkephalins: endogenous analgesics with an emerging role in stress resilience. Neural Plast 2017:1546125. https://doi.org/10.1155/2017/1546125
Pasternak GW (2018) Mu opioid pharmacology: 40 years to the promised land. Adv Pharmacol 82:261–291. https://doi.org/10.1016/bs.apha.2017.09.006
Valentino RJ, Volkow ND (2018) Untangling the complexity of opioid receptor function. Neuropsychopharmacology 43(13):2514–2520
Ironside M et al (2018) Brain mechanisms mediating effects of stress on reward sensitivity. Curr Opin Behav Sci 22:106–113
Manninen S et al (2017) Social laughter triggers endogenous opioid release in humans. J Neurosci 37(25):6125–6131
Hua S (2016) Neuroimmune interaction in the regulation of peripheral opioid-mediated analgesia in inflammation. Front Immunol 7:293
Brack A, Rittner HL, Stein C (2011) Immunosuppressive effects of opioids—clinical relevance. J Neuroimmune Pharmacol 6(4):490–502
Cechova K et al (2018) Up-regulation of μ-, δ-and κ-opioid receptors in concanavalin A-stimulated rat spleen lymphocytes. J Neuroimmunol 321:12–23
Roy S et al (2011) Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol 6(4):442
Mørch H, Pedersen BK (1995) β-Endorphin and the immune system-possible role in autoimmune diseases. Autoimmunity 21(3):161–171
Zhang C et al (2015) Beta-endorphin cell therapy for cancer prevention. Cancer Prev Res 8(1):56–67
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
N/A.
Informed consent
N/A.
Registry and the registration no. of the study/trial
N/A.
Animal studies
N/A.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oshaghi, M., Kourosh-Arami, M. & Roozbehkia, M. Role of neurotransmitters in immune-mediated inflammatory disorders: a crosstalk between the nervous and immune systems. Neurol Sci 44, 99–113 (2023). https://doi.org/10.1007/s10072-022-06413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06413-0