Abstract
The first case of coronavirus illness was discovered in Wuhan, China, in January 2020 and quickly spread worldwide within the next couple of months. The condition was initially only linked with respiratory disorders. After the evolution of various variants of the SARS-CoV-2, the critical impact of the virus spread to multiple organs and soon, neurological disorder manifestations started to appear in the infected patients. The review is focused on the manifestation of various neurological disorders linked with both the central nervous system and peripheral nervous system. Disorders such as cytokine release syndrome, encephalitis, acute stroke, and Bell’s palsy are given specific attention and psychological manifestations are also investigated. For a clear conclusion, cognitive impairment, drug addiction disorders, mood and anxiety disorders, and post-traumatic stress disorder are all fully examined. The association of the SARS-CoV-2 with neurological disorders and pathway is yet to be clear. For better understanding, the explanation of the possible mechanism of viral infection influencing the nervous system is also attempted in the review. While several vaccines and drugs are already involved in treating the SARS-CoV-2 condition, the disease is still considered fatal and more likely to leave permanent neurological damage, which leads to an essential requirement for more research to explore the neurological toll of the COVID-19 disease.
Similar content being viewed by others
Introduction
The global lockdown for several months in the age of space exploration is a big flag raised by a microscopic virus. The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) that causes the coronavirus illness has already earned a place in the history of fatal epidemics. By 16 December 2021, COVID-19 has already acclaimed 271,376,643 reported cases and took 5,324,969 lives, securing its place as one of the deadliest pandemics in the recorded human history [1]. The SARS-CoV-2 is highly susceptible to the mutations, and considering the fact, various variants of the coronavirus have come to participate, which are more transmissible and lethal and show a variety of new symptoms which were absent in the parent strain [2]. The COVID-19 disease is associated with neurological damage, which is likely to increase the chance of fatality and permanent neurological consequences on the infected patients. The further diversification of SARS-CoV-2 will create havoc and put a fatal blow on human society. In a recent case series of 214 individuals, 36.4% were found to be linked with symptoms in the central nervous system (CNS), 8.9% with symptoms in the peripheral nerve system (PNS), and 10.7% with symptoms in the skeletal system, which is an alarmingly high level [3]. Given the high possibility of the third wave of the pandemic in various nations, it is crucial to consider the scenario with a large number of deaths and the even higher number of incapacitations will be affiliated with the COVID-19 pandemic. The deployment of the required resources is essential to face these difficult times while saving as much lives as possible.
A critical point of focus concerning the management of the COVID-19 pandemic has been the surveillance of the new SARS-CoV-2 variants, of which some have been variants of concern (VOC) [4]. The emergence of alpha, beta, delta, and, very recently, omicron variants of concern (VOCs) have and are linked with surge in infections leading to different waves of infections around countries or sometimes globally also [5, 6]. The emergence of new variants is already stirring feelings of anger, uncertainty, and frustration in people amidst the pandemic which is adversely affecting mental health and their social and economic well-being which acts a breeding ground for various neuropsychiatric illnesses. The neurological, cognitive, and psychiatric sequelae of specific SARS-CoV-2 variants are understudied, and in light of the increasing number of patients with such sequelae post COVID-19 infection, there is a pressing need to study the neurological toll of COVID-19 variants.
Nature of SARS-CoV-2
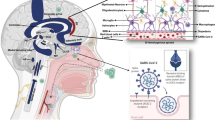
SARS-CoV-2 is a single-stranded positive-sense RNA virus that causes respiratory viral illness and shares 96% of its genome with Rhinolophus affinis RaTG13 (horseshoe bat virus) [7]. Spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N) are the four structural protein genes in the genome [8]. The spike protein gene produces a homo-trimeric, type I fusion, and transmembrane glycoprotein that aids the virus’s invasion of the host cell’s angiotensin-converting enzyme-2 (ACE-2) receptors [9]. ACE-2 receptors are found in 85 distinct types of human tissues such as the type II pneumocytes, epithelial cells of lungs, and endothelial cells of blood vessels, and 21 of them are in various brain regions such as the amygdala, cerebral cortex, and brainstem [10, 11]. The receptor-binding domain (RBD) region contains the core structure and a variable receptor binding motif, which interacts and attaches itself with the ACE-2 receptor of the host cell [12]. The invasion of SARS-CoV-2 by the interaction with the ACE-2 is based upon the “Anchor-Locker” mechanism in which β-sheet (1, 2) and loops (1, 2, and 3) play a vital role in the complete transition process. Loop 2 is involved in the recognition and binding of ACE-2 and acts as “Anchor”; on the other hand, loop 3 stabilizes the whole structure and acts as a “Locker” in this complete mechanism. After SARS-CoV-2 and ACE-2 have been recognized, β-sheet 1 works to strengthen and improve the binding [13]. The priming of SARS-CoV-2’s spike protein by the host cell is critical for the entry of the virus. This interaction with the ACE2 determines the efficiency of SARS-CoV-2 transmissibility [10]. A simple understanding can be acquired by taking an outlook over Fig. 1.
Possible pathway to the brain
SARS-CoV-2’s exact route and mechanism of action are yet unknown, which opens the door to various possible hypotheses which can come into play. As mentioned earlier, various cells such as glial cells and neurons contain ACE-2 receptors which makes the brain a target of SARS-CoV-2 [11]. The genetic material and various proteins of the viruses have been found in the brain tissue of the patients, which gives a hint towards the neural pathway as a possible route for the virus to get inside the brain [14, 15]. The after effects of the viral infection may cause the disruption of the nasal epithelium, which may help the virus to enter into blood vessel or lymph vessels to infect various organs, including the brain [16]. ACE-2 receptors are also found in the capillary endothelium, and the interaction with the virus may lead to disrupting the blood–brain barrier by allowing viral access into the central nervous system [14, 17]. Several cases of loss of smell in the infected patients are already reported, which leads to understanding the fact that the virus can penetrate the olfactory mucosa. Another possible route for the virus would be to travel through cribriform plate or through trigeminal or vagal nerve [18]. Virus can also invade by infecting the motor or sensory nerves [19]. SARS-CoV-2 can also attempt to reach the central nervous system through circumventricular organs, such as midline part around the third and fourth ventricles. This part is responsible to monitor the blood and cerebral spinal fluid, and the capillaries lack the junctional protein which is normally present in the blood–brain barrier. Reverse transcription using polymerase chain reaction detects viral RNA, but in situ hybridization in the medulla and cerebellum yields no results [20].
The growth of SARS-CoV-2 in lung tissue cells affects alveolar gas exchange, resulting in severe CNS damage such as hypoxia and an increase in anaerobic metabolism in the brain [21]. The virus infects the macrophages and other cells involved in the nervous system, which in response activates the glial cells and cause the inflammatory response syndrome [14]. Acute necrotizing encephalopathy can also be caused by cytokines crossing the blood–brain barrier [22]. Instead of direct attack, it is quite feasible for the SARS-CoV-2 to get help from the proteins already present in the host body to enter the nervous system. It is being considered that the virus can bind to human protein neuropilin-1 (NRP-1) which may allow the viral invasion as NRP-1 support the entry of particles into the CNS that are similar in size to SARS-CoV-2 [23]. The viral RNA found in the leptomeninges and Virchow-Robin gaps can induce contamination through blood vessels. Despite this, histopathological examination showed no link between microglial nodule levels and neuron phagocytosis in the brain and the levels of viral messenger RNA found [24]. For better understanding, take an outlook over Fig. 2.
Standard neurological symptoms
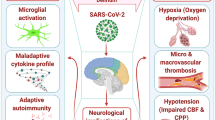
The SARS-CoV-2 is reported to exhibit and influence the neurotropic properties, which are likely to be linked with serious neurological conditions and may leave permanent damage [21]. Out of those disorders, some of the commonly found is acute cerebral infraction or hemorrhage, while neurological symptoms such as headache, myalgia, nauseas, and loss of consciousness are also reported to be found in a major infected population [14]. The cases of brain damage caused by the COVID-19 disease are reported to have both macro- and micro-hypoxic or ischemic injuries and infracts, giving possible conclusion to the necrosis of brain tissue. The complement cascade signifies the synaptic pruning by microglia as post-viral infection response [18, 24]. The overall mechanism of brain damage caused by the COVID-19 disease seems to resemble the mechanism of the traumatic brain injury in which the neuronal loss is related to the pathogenesis of suicidal behavior by the proinflammatory response and microvascular injury [25]. While the actual mechanism is yet to be understood completely, intensive research is required in association with neural tissue to resonate a plausible explanation of the neurological symptoms. Various neurological manifestations reported in the coronavirus-infected patients are mentioned in Table 1.
Headache
Headache is considered as the most common COVID-19-related neurological manifestation. At present, definite estimates of COVID-19-related neurological symptoms are not available. However, there is mounting evidence that SARS-CoV-2 has an impact on the brain and other organs. The majority of COVID-19 patients have a headache that is accompanied by a temperature. It has been recorded in almost 6.5–34% of patients [26, 27]. The intensity is mostly described as mild [28]. The occurrence of headaches in SARS-CoV-2 is often associated with the cytokine storm [29, 30]. More studies would be required to validate this relationship. It has been suggested that ibuprofen can enhance ACE2 expression, which could make SARS-CoV-2 infection worse [31]. However, another study does not support this statement [32]. As a result, there is presently no evidence to support the use of nonsteroidal anti-inflammatory medications (NSAIDs) in COVID-19 headache sufferers.
COVID-19 risk is higher in individuals with concomitant migraine due to angiotensin system and NLRP3 inflammasome-mediated processes connected to vascular and inflammatory comorbid diseases, especially as people age. The NLRP3 inflammasome complex’s involvement and pericyte dysfunction are both crucial in SARS-Cov-2-related pathogenesis and may play a role in migraine neuro-vasculo-inflammatory processes [33]. More research needs to be done on investigating how COVID-19 affects patients with migraine.
Chemosensory impairment
In a high number of COVID-19 patients, chemosensory dysfunction (CD) is discovered. Anosmia (partial or full loss of scent), hyposmia (decreased perception of smell), ageusia (partial or complete loss of taste), and hypogeusia are all common symptoms of CD (reduced ability to taste things). However, the pathogenesis of chemosensory impairment related with COVID-19 is poorly elucidated at present [34, 35].
Anosmia is also reported in cases of influenza [36]. In a research done by Yao et al. in 2020, 1480 individuals with influenza-like symptoms were tested for COVID-19 between March 3, 2020, and March 29, 2020, with 58% of patients testing positive for COVID-19 and 15% testing negative. COVID-19-positive patients reported losing their sense of smell and taste 68% and 71% of the time, respectively, compared to 16% and 17% of COVID-19-negative patients. COVID-19 was shown to be related to smell and taste impairment independently and strongly, but not to sore throat [37].
Mao et al. in 2020 reported in Wuhan, China, that only 5.1% and 5.6% of COVID-19 patients reported smell and taste impairment, respectively [3]. Chemosensory impairment is also reported in other coronavirus infections; however, it is not accompanied by nasal obstruction or rhinitis. SARS-CoV-2 is believed to directly damage the gustatory and olfactory receptors [38]. There is not any evidence on the definite time frame for patients to regain their sense of taste or smell. CD is also one of the earlier symptoms of COVID-19; dedicated anosmia testing may offer the possibility of early detection of COVID-19 infection [36].
Psychosis
A higher incidence of COVID-19 individuals with more severe illness have mental symptoms. Psychosis is a relatively lesser reported neuropsychiatric symptom in COVID-19 patients. The neurobiology governing the incidence of psychosis in COVID-19 patients is not clear at present. However, stressors like the fear of death from the infection, social isolation, lack of support, and psychosis is thought to be more common in COVID-19 patients due to stress and financial strain [39].
The coronavirus being neurotropic is said to affect limbic structures that govern behavior. Because many difficulties like medicine compliance, lack of review, poor awareness, and stress diathesis are involved with any biological disaster, it is difficult to conceive a clear relationship between an increase in pre-existing psychiatric disorders and the virus’s neurotropic effects [40].
Coronaviruses and human respiratory viruses are considered to be opportunistic and underestimated because of their neuro-invasive qualities either due to viral replication or autoimmunity [41]. The psychological context and burden of the disease can fuel the morbidity of existing psychiatric disorders in COVID-19 patients [42]. Immune-based triggers have also been used to explain the occurrence of psychiatric disorders like depression, anxiety, schizophrenia, psychosis, and neuropsychiatric manifestations of various viral diseases like the human immunodeficiency virus (HIV) [43].
Sleep disturbances
Sleep is crucial for the control of psychological and physiological functions [44]. Stress, depression, and anxiety due to COVID-19 have led to the increase of prevalence in sleeping disorders. Alimoradi et al. in 2021 reported that in the general population and healthcare workers, sleep difficulties were predicted to affect 37% of people [45]. A review of the medical records of 329 COVID-19 patients revealed that 25.5% had psychiatric consultations; 33% had sleep disorders such as insomnia, early awakening, and difficulty falling asleep; and 22.6% and 54.8% were prescribed benzodiazepines and nonbenzodiazepine sedative-hypnotics, respectively (zolpidem) [46].
However, the incidence of sleep disorders in patients with active COVID-19 was higher. COVID-19’s psychologically distressing effects may cause sleep problems as well. Due to an abnormal innate immune response, SARS-CoV-2 can cause secondary neuronal injury, which leads to sleep, mood management, pain sensitivity, and energy levels all affected as a result of the chronic neurological sequelae [47]. Other physiological causes include SARS-CoV-2 spreading fast to certain brain regions, such as the thalamus and brain stem, which play significant roles in sleep management and respiratory regulation, raising the likelihood of aberrant sleep–wake behavior and SDB [48]. Environmental factors include noise, abnormal light exposure, patient care activities, and diagnostic and therapeutic procedures, and other variables may contribute to ICU-related sleep disruption [47].
COVID-19 has a negative influence on sleep and affects everyone, including the general public, healthcare personnel, and COVID-19-afflicted patients. COVID-19’s prognosis is worsened by sleep disruptions, poor mental health, and deteriorated physical condition caused by SARS-CoV-2 infection. It is the need of the hour to devise group-specific strategies to address sleep disturbances [47].
Disorders linked with cerebral nervous system (CNS)
Acute stroke/cerebrovascular disease (CVD)
Cerebrovascular accidents more commonly known as acute stroke is linked with poor blood flow to the brain, which is likely to cause serious damage to the brain tissue. Most common types of acute stroke are ischemic and hemorrhagic. Acute ischemic stroke (AIS) is caused by the lack of blood flow and intracerebral hemorrhagic (ICH) is due to bleeding. Even though the COVID-19 disease is already linked with other organs other than the respiratory system, the link between SARS-CoV-2 and CVD is yet to be clearly established [49]. SARS-CoV-2 is a virus that infects people and is likely to increase the chances of thrombotic vascular events and by 7.6-folds in comparison to influenza [50]. Coronavirus disease is found to have association with increased prevalence of large artery occlusion, infraction, and cryptogenic etiologies [49]. Large artery occlusion is likely to be caused by cardio-embolism or paradoxical embolism in case of coronavirus disease [51]. In a case series of 219 patients infected by COVID-19, 4.6% of them have developed ischemic stroke. The infected patients are likely to be associated with higher inflammatory response than the patients with the similar disorder but not infected with SARS-CoV-2 [52]. Ischemic stroke is likely to be the most common type of stroke and frequently found [49].
The mechanism of CVD seems to be influenced by various factors, and the role of SARS-CoV-2 is to act as the trigger instead of involved in the direct invasion. The specific pathophysiological mechanism of AIS and ICH could be directly caused by SARS-CoV-2 [49]. Elevated D-dimer and fibrinogen are linked with sepsis-induced coagulopathy (SIC), which is responsible for the infection-induced systemic inflammatory response and may lead to thrombosis and stroke [53, 54]. The viral infection can be linked with the consumption coagulopathy, which causes fibrinogen depletion and increases the risk of ICH [55], while the presence of ICH is quite rare in comparison to the AIS. The interaction of the SARS-CoV-2 virus with the ACE-2 receptors may disrupt the integrity of intracranial arteries and cause vessel wall rupture [56]. This interaction can also limit the number of accessible receptors, resulting in renin angiotensin system (RAS) downregulation [53]. Downregulation of RAS can raise blood pressure, putting hypertensive patients at an even greater risk of hemorrhagic stroke [56]. It has been established that 1.4% of COVID-19-infected individuals have developed acute CVD.
Cerebral venous sinus thrombosis (CVST)
The presence of a blood clot in the dural venous sinuses, cerebral venous thrombosis, or both characterizes cerebral venous sinus thrombosis (CVST). Despite the fact that numerous cases of CVST have been correctly identified in COVID-19-infected individuals, knowledge of the SARS-CoV-2 and CVST connection is still restricted [57]. The likeliness of the CVST cause may involve the depletion of the available ACE-2 receptors, which make ACE1 unopposed by the production of the angiotensin II and promote the thrombosis [58]. The existence of anti-phospholipid antibodies in highly infected individuals, such as IgA anticardiolipin antibodies and IgA and IgG beta 2 glycoprotein I antibodies, would be the most apparent indication of coronavirus illness in connection with the CVST [49, 58]. The prevalence of the CVST is likely to be caused by the hyperinflammatory and hypercoagulable state such as SIC in association with the SARS-CoV-2 infection [59]. In a case study involving 28 patients, the mortality rate of CVST associated with the COVID-19 is calculated to be 35.29% [57]. In a reported case of COVID-19-infected CVST, the patient had headache which leads to right-sided weakness, numbness, and expressive aphasia and concluded to be sigmoid and transverse sinus thrombosis [60].
Encephalitis
Encephalitis also known as the acute brain inflammation is generally linked with the viral infection, making the host quite vulnerable to this disorder while facing SARS-CoV-2. In a patient having clinical meningoencephalitis, the genetic material of the SARS-CoV-2 was found in the cerebrospinal fluid [61]. Encephalitis gives a clear indication towards the invasion of the coronavirus, while other possibilities of encephalitis are also there such as SARS-CoV-2-mediated cytokine storm syndrome, which can be linked with different severe symptoms. Yet the presence of the encephalitis is found to be linked with the altered mental status in a COVID-19 patient [22].
Acute necrotizing encephalopathy (ANE) is a rare type of complication of viral infection linked with necrosis in CNS and presence of multiple areas of edema. This particular disorder is also associated with the uncontrolled release of the cytokine during disease such as influenza, common flu, and in this case coronavirus disease [22, 62]. It is found quite plausible for ANE to play a role in disruption of blood–brain barrier without direct invasion of the SARS-CoV-2, which provides another possible explanation of the neurological disorder manifestations even without direct invasion in the neuron [22].
Another COVID-19-associated disorder would be the limbic encephalitis. In a COVID-19-infected patient, cerebrospinal fluid lymphocytic pleocytosis was diagnosed, linked with the anti-Caspr2-associated limbic encephalitis [63]. Despite that, the mystery of the role of virus in manifestations of encephalitis remains unsolved due to absence of any strong evidences.
Delirium
Delirium is a severe neuropsychiatric syndrome involved with the acute deficits in attention and cognition processes. Delirium is often linked with the altered arousal, lack of responsiveness, hypervigilance, psychosis, delusions, hallucinations, and frequent mood swings [64]. In a recent case study of 58 patients, 65% of them had regularity of delirium [65]. The mechanism behind the manifestations of the delirium is still unclear, but the plausible cause seems to be similar to other disorders such as the invasion in CNS, induction of immune response, or secondary effects of involvement of different processes. Irrespective of the process, delirium seems to be found quite frequently in the infected patients. COVID-19 patients are considered to be at higher risk due to the prolonged isolation; in some early reports, 7.5% of the infected patients are diagnosed with the delirium-like symptoms, but the case of delirium is highly undervalued as almost 75% of patients are not given special attention unless the patient is properly evaluated and has medical history for delirium [66].
Parkinson’s disease (PD)
Parkinson’s disease (PD) is a neurodegenerative condition that affects people for a long time involving the CNS and especially affects the motor system. In several studies, it has been reported that the individuals suffering from PD are likely to be more vulnerable to the neurological disorder manifestation in COVID-19 disease [67, 68]. In a recent meta-analysis, the hospitalization rate for the PD patients infected with SARS-CoV-2 is calculated to be 39.89%, while the mortality rate to be 25.1%. Even the prevalence and severity of the COVID-19 disease including the mortality are considered to be higher than the general public [67]. Other disorders are found more frequent in the PD patients such as dyspnea, which is a respiratory dysfunction due to the muscle weakness and inadequate respiration excursion and found in 39% of the patients [69]. PD patients are likely to have impaired mastication and swallowing reflexes, which can lead to aspiration pneumonia. To make the scenario even more critical, the patient is likely to suffer from neurodegeneration in the medulla’s respiratory center [70].
Parkinsonian hyperpyrexia syndrome (PHS) is a movement disorder which is also found in SARS-CoV-2-infected PD patients, which is likely due to fever and altered dopaminergic medications [71].
Opsoclonus-myoclonus-ataxia syndrome (OMAS)
Opsoclonus-myoclonus-ataxia syndrome is a rare CNS autoimmune disease linked with the peculiar movement of eyes and limbs and abnormal behavior is often present. OMAS is made when different characteristics are present together such as opsoclonus, myoclonus, ataxia, sleep disturbance, anti-neuronal antibodies [72, 73]. Opsoclonus is the involuntary movements of the eye; myoclonus is associated with the paroxysmal and involuntary contraction of the muscle for short duration and caused by bacterial, viral, or parasitic infection; and ataxia of the other hand is characterized by the loss of muscle control and coordination [72]. Being immune mediated, the prevalence of the OMAS can be associated with the immune-mediated mechanism, which is likely to be the result of the immunotherapies present to treat COVID-19 infection [73]. Another possibility of OMAS would be the disruption in the saccade, cerebral, and motor circuits due to the hyperexcitation of the brainstem. Hyperexcitation can be caused by the dysfunctional Purkinje cells due to the presence of the different antibodies present in the nervous system [72,73,74]. However, due to the lack of data and explicit research, the involvement of SARS-CoV-2 and the OMAS is not clear.
Cytokine release syndrome
In various studies, it has been found that SARS-CoV-2 can cause serious damage to the CNS by promoting the inflammatory cytokine production such as IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, and IFN-γ. This excess release of cytokines is called cytokine release syndrome or cytokine storm syndrome; the neurotropic viruses induce IL-6 production from glial cells; release of IL-6 stimulates the macrophages in the brain. Due to the association of the glial cells, this interaction may lead to chronic inflammation and serious brain damage [21, 75]. The cytokine release syndrome can be the cause of multiple organ failure, which increases the possibility of the mortality in the COVID-19 infection [76]. The presence of cytokines will promote atherosclerosis, plaque, rupture, stroke, and thrombosis [28]. This scenario combined with the endothelial injury will promote the tissue factor expression and worsen the thrombotic state [77]. The cytokine storm syndrome gives SARS-CoV-2 the capability to indirectly inflict damage to the host’s nervous system without any invasion in the CNS, which is likely to increase the mortality rate significantly.
Disorders linked with peripheral nervous system (PNS)
Bell’s palsy
Bell’s palsy, often known as paralysis, is a sudden-onset peripheral facial nerve palsy that has been linked to SARS-CoV-2 virus in some cases [78]. In a recent analysis, the prevalence of Bell’s palsy in COVID-19-infected patients is found to be 0.08% and recurrent to 8.6% to those who were diagnosed with Bell’s palsy prior to the infection [79]. The proposed mechanism of Bell’s palsy resembles with the other neurological disorders such as such as CVD and AIS. The SARS-CoV-2 RNA was not found in the cerebrospinal fluid in any of the reported cases [80]. The accurate mechanism is not clear yet and requires dedicated research and pathological analysis. In a case report of facial nerve palsy, a left-sided facial weakness developed in a patient infected with coronavirus disease, which led to left retro-auricular pain and dysgeusia. And after 1 week, there was no subtle improvement in the case of Bell’s palsy [78].
Rhabdomyolysis
Myositis more commonly known as muscle inflammation is reported in various cases linked with coronavirus disease [80]. The severe variant of myositis would be rhabdomyolysis, which is characterized by the infraction of skeletal muscles, release of toxins, and elevated myoglobin, creatine kinase, aldolase, and lactate dehydrogenase. It can lead to kidney failure and intravascular coagulation and can be fatal [80, 81]. Rhabdomyolysis is linked with autoimmune myopathy and can also be caused by substance abuse, viral infection, seizure, and strokes. Influenced by SARS-CoV-2, the prevalence of rhabdomyolysis can be the result of the myocyte injury, cytokine storm syndrome, and direct damage by the viral toxins. However, the accurate mechanism in association with SARS-CoV-2 is not clear yet [81].
Guillain–Barre syndrome (GBS)
Guillain–Barre syndrome is a demyelinating polyneuropathy, meaning the autoimmune response to the peripheral nervous system that manifests as acute ascending paralysis. It is characterized by the facial weakness and neuropathy [82]. The variant of the GBS would be the Miller-Fisher syndrome (MFS), which is linked with the gait ataxia, areflexia, and ophthalmoplegia. Both GBS and MFS are discovered to be linked to SARS-CoV-2 [83]. The GBS is characterized by signal hypersensitivity, nerve root and cauda equina augmentation, and chronic inflammatory demyelinating polyneuropathy [83, 84]. In reported case of GBS, where the patient was infected with COVID-19 disease, the patient developed progressive bilateral ascending paralysis 2 weeks after the first respiratory symptom appeared and no outcome was reported after the treatment [85].
Psychiatric sequel of COVID-19
Given the CNS and PNS symptoms induced by the viral infection, the link between SARS-CoV-2 infection and mental disorder has been highly worrisome [86]. The coronavirus disease represents a neurological, psychiatric, and cognitive sequela. Despite being primarily a respiratory tract illness, COVID-19 has been found to have substantial mental health consequences, since social isolation can exacerbate psychiatric symptoms, which are frequently associated with other neurological and neuropsychiatric disorders [87, 88].
Our understanding of COVID-19 is rapidly evolving. There is more emerging data highlighting the neurological, psychiatric, and COVID-19’s cognitive aftereffects. Due to the complexity of COVID-19 pathogenesis, it is essential to focus on building multidisciplinary studies to improve our understanding of clinical trajectory of the occurrence of psychiatric disorders followed by SARS-CoV-2 [89]. There has been extensive research done on the acute clinical manifestations and etiology of COVID-19. However, the psychiatric manifestations of COVID-19 have been understudied. A greater knowledge of COVID-19’s mental symptoms and consequences might lead to improved therapeutic care [90]. At present, the clinical management of COVID-19 involves containing and targeting pulmonary manifestations. This section of the review aims to highlight the psychiatric sequel of COVID-19 in patients.
Anxiety, sadness, sleeplessness, drug addiction, and post-traumatic stress disorder are among the most prevalent mental symptoms described by recovered patients (PTSD, which also is a type of an anxiety disorder). It is also believed that the unpredictability and psychosocial implications of the virus also add to the mental dimension of the disease, which certainly needs more attention from experts. It has also been noted that unmitigated neuroinflammation in COVID-19 has also been the culprit behind a range of neuropsychiatric manifestations (like depression and anxiety) as it has been in the case of severe pulmonary complications caused by the disease. Many psychiatric disorders are believed to be caused by these immune-inflammatory states [86].
Upon SARS-CoV-2 infection, the immune system upon activation releases cytokines. They are known to affect the metabolism of neurotransmitters like serotonin, dopamine, and glutamate through their synthesis, release, and reuptake [91]. A disruption in any of the listed neurotransmitters can cause depression, delirium, and memory loss. COVID-19 patients with existing psychiatric comorbidities have lessened immunity. Upon infection, their illness gets aggravated by the virus. In 2021, Nakamura et al. reported that over 30% of COVID-19-hospitalized patients had cognitive impairment, sadness, and anxiety that lasted several months after release. The incidence of psychiatric disorders is seen to increase in patients with more severe COVID-19 symptoms [92]. Another concern of opioid use and disability management post-acute COVID-19 recovery still remains a matter of concern among pain management specialists [89, 93]. Finally, clinical trials are urgently needed to establish the optimal treatment options for COVID-19’s neuropsychiatric and other possible long-term consequences.
Until recently, we have assumed that psychiatric disease is both a cause and a result of COVID-19. In a retrospective cohort research based on electronic health records of 6-month neurological and psychiatric outcomes in 236,379 COVID-19 survivors, Taquet et al. (2021) found a significant neuropsychiatric and mental morbidity 6 months after infection with COVID-19. In the next 6 months after infection, 33.62% of 236,379 COVID patients were diagnosed with a neurological or mental illness. Of those surveyed, 12.64% obtained a diagnosis of this kind for the first time [94].
In this section, we aim to describe post-COVID-19 psychiatric manifestations that are majorly observed:
Cognitive impairment
There have been certain studies pointing towards the incidence of cognitive impairment in both acute and chronic COVID-19 patients. In a meta-analytical analysis published in 2020, Roger et al. identified the negative impacts of SARS-CoV and MERS-CoV infections on patient cognition. Almost a quarter of those who were infected showed cognitive problems months or years later [95]. After hospital release, 34% and 28% of COVID-19 patients experienced memory loss and poor concentration, respectively, in a study of 279 hospitalized COVID-19 patients. [96]. According to Taquet et al.’s study, the rate of new-onset dementia after hospitalization in COVID-19 was 2–3 times higher than that of hospitalization for other medical reasons [94].
Long-term cognitive impairments have been documented as a result of delirium experienced during acute illness in more severe COVID-19 patients [92]. One of the most prevalent mental symptoms in COVID-19 patients is delirium. Delirium has also been documented in COVID-19 individuals who did not have any other serious medical issues [97, 98]. Instances of “brain fog” (not a medical diagnosis, but it refers to problems with thinking, memory, and concentration) have been reported by patients with milder symptoms who did not need hospitalization, and possibly never had delirium [99]. Patients experiencing COVID-19-related cognitive impairment should benefit from longitudinal cognitive assessment and self-report measures [100, 101].
Substance abuse disorders
With an increased incidence of substance abuse with the surge of COVID-19 cases across the globe, medical screening for drug addiction problems has been an important part of COVID-19 participants’ follow-up care. Due to societal and public constraints, patients who are currently misusing drugs are more likely to get COVID-19. Many people experience alarming withdrawal symptoms as a result of social isolation, limited celebrations, and the inability to obtain addictive substances. While most outdoor activities have been phased out in most areas, a major percentage of the population is turning to binge watching television and spending a significant amount of their day on electronic devices, setting the framework for the development of behavioral inclinations [87].
In COVID-19 patients with concomitant drug abuse disorders, there was a higher rate of hospitalization (40.1% vs 30.1%) and mortality (9.6% vs 6.6%) compared to patients without a history of substance misuse [102]. Use of illicit substances especially opioids is known to severely damage pulmonary function [103]. Patients with COVID-19 with concomitant drug misuse are more likely to develop severe symptoms because they are sensitive to COVID-19 risk factors such as obesity, type 2 diabetes, CVD, cancer, chronic liver, and kidney disease [102, 104]. Additionally, reduced immune function as a result of complicated opioid immune regulation, as well as pharmacological interactions between opioid use disorder medicines and COVID-19, are molecular reasons for worse outcomes in this susceptible population of COVID-19 patients [105].
Mood and anxiety disorders
There is enough evidence to highlight the occurrence of depression and anxiety with COVID-19 [106]. Anxiety and depressive symptoms rather than the occurrence of the anxiety or depressive disorder have also been observed in mild cases [92]. The rates differed based on the population examined, the techniques used to measure symptoms, and the period after infection symptoms were assessed.
A study conducted in New York City by Parker et al. in 2020 indicated symptom severity and hospitalization to be key drivers in increasing the occurrence of depression and anxiety in COVID-19 patients. During hospitalization, 36% of participants reported anxiety and 29% reported depression; 14–17 days later, anxiety and depression prevalence had dropped to 9% and 20%, respectively, although acute stress symptoms had developed in 25% of individuals [107].
Prior mental disorders, gender, individuals with infected family members, post-infection physical pain, increased inflammatory markers, and other risk variables are among the most important. Suicidal inclinations have been noted in COVID-19 patients. Several stories have surfaced of COVID-19 patients attempting suicide during or before their hospitalization [108, 109]. It is best to wait until comprehensive epidemiological research on the link between COVID-19 and suicide are finished before making a final decision [92]. After SARS-CoV-2 infection, there are no well-defined pharmacologic recommendations for treating sadness and anxiety. However, the basic strategy to managing mental symptoms in the medically unwell may be extended to this population as well [92, 110].
Post-traumatic stress disorder (PTSD)
Anxiety disorders such as post-traumatic stress disorder (PTSD) are a kind of anxiety condition that is characterized by flashbacks, nightmares, emotional numbing, and serious anxiety that are typically followed by a traumatic event like death, serious violence, sexual or physical assault, or a combat. It is believed that the incidence of PTSD among COVID-19 survivors is critically high [111]. In 2021, Chamberline et al. published a research that looked at the prevalence of PTSD in 13,049 survivors of suspected or confirmed COVID-19. They discovered that PTSD ratings differed a lot. This is because those with more severe respiratory distress have greater levels of PTSD than people who do not have any respiratory symptoms (no respiratory symptoms assistance) [112].
Currently, the incidence of PTSD appears to vary between 20 and 30% among COVID-19 patients, whereas the prevalence of less precisely characterized post-traumatic stress symptoms (PTSS) varies significantly [92]. In contrast, 20.3% of 64 Korean COVID-19 patients questioned 2.5 months after being discharged from the hospital fulfilled PTSD criteria [113].
By reviewing available literature on the occurrence of PTSD following COVID-19 infection have aided our understanding of the risk factors for the same. They include younger age, gender, comorbid psychiatric condition, and severe respiratory symptoms leading to hospitalization and ICU care. Interestingly, it has been noted that patients with COVID-19 in the ICU having obesity had an increased risk of PTSD, but this relation has not been observed in any other medical condition [114]. At present, COVID-19-related PTSD/PTSS-specific pharmacological interventions have not been studied extensively by taking consideration of different variable.
Preventive measures, treatment remedies, and immune invasion
The COVID-19 pandemic has already taken millions of lives, and protection against the invasion is critically important. Being a respiratory viral infection, the basic precaution steps include wearing masks in public places, regular washing or sanitation of hands, and maintaining social distancing, which means to have at least 3 feet of distance between two individuals. Avoid any crowded places, going out unless absolutely necessary, public transport, gathering, and public places. Following the guidelines from the health authority is absolute and take mandatory steps to prevent the coronavirus disease and make sure to get vaccinated [115].
The traditional treatment for the mild to moderate coronavirus disease involves the monoclonal antibodies such as bamlanivimab, etesevimab, and REGN-CoV2. The authorized antibody cocktail dose is comprised of 700 mg bamlanivimab and 1400 mg etesevimab [116]. Regdanvimab (CT-P59), a monoclonal antibody, binds to the receptor-binding domain of spike protein and blocks the interaction between RBD and ACE-2 but blocking the cellular entry. Regdanvimab is found to be capable to neutralize the delta variant effectively in pre-clinical in vivo studies [117]. Other than antibody treatment, typical drugs are also prescribed actively. One of the most recommended antiviral drug for coronavirus disease is remdesivir, which is involved in inhibiting the viral RNA-dependent RNA polymerase, and prescribed for patients with severe symptoms [118]. While allopathic drugs are in action, the traditional medicines also played a major role in the treatment of SARS-CoV-2-infected patients. In China, 85% of the infected patients were treated with the traditional medicines such as extract of Isatis indigotica and Houttuynia cordata [118, 119]. The most commonly used drugs to treat SARS-CoV-2 can be seen in Table 2 [120].
By 20 August 2021, 32% of the world population has been vaccinated with at least one dose of vaccine against SARS-CoV-2 irrespective of the brand name and 24% are fully vaccinated [121]. Currently current vaccinations are either mRNA-based, adenovirus vector–based, or inactivated vaccines. The genetic code for a single antigen of the virus is included in the mRNA-based vaccination, allowing the receiver to produce antibodies against that antigen. While adenovirus vector vaccines include the DNA of the spike protein antigen, the modified virus acts as a vector, allowing the antigen to be expressed and antibodies to be produced again. Inactivated vaccines are made up of inactivated viruses that have been exposed to chemicals, heat, or radiation in order to serve as antigens [120]. The Pfizer-BioNTech mRNA vaccine is found effective against the alpha variant [122]. Johnson and Johnson vaccine is also compelling in stimulating the protective neutralizing antibodies [123]. Different vaccines which are in circulation are mentioned in Table 3 [120].
Despite that, the new variants seem to have found the way to escape from the monoclonal antibodies, it seems that the evolution of intermediate mutants which are flowing and targeting the immunocompromised patients where the possibilities of viral replication is higher while on going treatment with immune plasma or monoclonal antibodies [124, 125]. The vaccines which are circulating in the vaccination program seem to lose their potency against the new variants due to their critical mutations in the RBD region. The affinity between the antibodies and the variants are likely to take a critical blow and likely to disrupt the antibody recognition towards the SARS-CoV-2, which may lead to higher immune escape [126]. The immune sera from the human vaccinated with Pfizer/BioNTech is significantly reduced in neutralizing titers against the alpha variant [127]. Another vaccine named ChAdOx1 from AstraZeneca devastatingly lost its protection against the beta variant to 10%, while it earlier showed 75% of protection against the alpha variant [128, 129]. This challenge of immune escape requires a different approach to deal with it, as it may not only lead to new fatal symptoms but make this coronavirus disease even harder to treat than it already is.
Conclusion
Millions of people have died as a result of the new coronavirus epidemic that has swept the globe. COVID-19, which is caused by SARS-CoV-2, has a wide pathophysiology and is linked to significant morbidity and death rates across the world. SARS-CoV-2 was primarily considered to be a respiratory virus; however, it has produced staggering and widely ranging cognitive, neurological, and psychiatric sequelae in patients, which further complicates clinical management. The incidence of cognitive, neurological, and psychiatric complications due to COVID-19 is being increasingly reported across different geographies. Further research should be directed to determine the neurotropic nature of the novel coronavirus disease; whether it initiates hypercoagulation or peripheral immune activation to deteriorate brain function would be pivotal to recognize and mitigate any neurological or psychiatric threats [130]. Furthermore, it is the need of the hour to direct more research towards the development of therapeutics to mitigate and treat the perilous neurological sequelae of COVID-19. Personalized ‘omics methods can aid in the identification of individuals who are at high risk of developing neurological symptoms. Biological variables like chronological age, gender, comorbidities, existing neurological or psychiatric conditions, and genetic history can be taken into account to predict the prognosis of COVID-19. It is also essential to comprehensively characterize the spectrum of neurological symptoms associated with the novel coronavirus disease to gain more sagacity on the breadth of COVID-19’s cognitive, neurological, and psychiatric complications. Hence, more unbiased population studies across larger geographies can give us a clearer picture of the etiology and incidence of the neurological sequelae of COVID-19 [97].
Abbreviations
- ACE-2:
-
Angiotensin-converting enzyme-2
- AIS:
-
Acute ischemic stroke
- ANE:
-
Acute necrotizing encephalopathy
- CD:
-
Chemosensory dysfunction
- CVD:
-
Cerebrovascular disease
- ICH:
-
Intracerebral hemorrhagic
- MFS:
-
Miller-Fisher syndrome
- NRP-1:
-
Neuropilin-1
- PHS:
-
Parkinsonian hyperpyrexia syndrome
- RBD:
-
Receptor-binding domain
- RAS:
-
Renin angiotensin system
References
(2021) WHO Coronavirus (COVID-19) Dashboard |. In: WHO. https://covid19.who.int/. Accessed 16 Dec 2021
Chan JF-W, Kok K-H, Zhu Z et al (2020) Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 9(1):221–236. https://doi.org/10.1080/22221751.2020.1719902
Mao L, Jin H, Wang M et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77(6):683–690. https://doi.org/10.1001/jamaneurol.2020.1127
Jewell BL (2021) Monitoring differences between the SARS-CoV-2 B. 1.1. 7 variant and other lineages. Lancet Public Health 6(5):e267–e268. https://doi.org/10.1016/S2468-2667(21)00073-6
Karim SSA, Karim QA (2021) Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 398(10317):2126–2128. https://doi.org/10.1016/S0140-6736(21)02758-6
Thakur V, Bhola S, Thakur P et al (2021) Waves and variants of SARS-CoV-2: understanding the causes and effect of the COVID-19 catastrophe. Infection 16:1–16. https://doi.org/10.1007/s15010-021-01734-2
Zhou P, Yang XL, Wang XG et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798):270–273. https://doi.org/10.1038/s41586-020-2012-7
Chan JF-W, Kok K-H, Zhu Z et al (2020) Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 9:221–236. https://doi.org/10.1080/22221751.2020.1719902
Biswas S, Thakur V, Kaur P et al (2021) Blood clots in COVID-19 patients: simplifying the curious mystery. Med Hypotheses 146:110371. https://doi.org/10.1016/j.mehy.2020.110371
Hoffmann M, Kleine-Weber H, Schroeder S et al (2020) SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181(2):271–280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Lukiw WJ, Pogue A, Hill JM (2022) SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol Neurobiol 42(1):217–224. https://doi.org/10.1007/s10571-020-00947-7
Wan Y, Shang J ,Graham R et al (2020) Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 17;94(7):e00127–20. https://doi.org/10.1128/JVI.00127-20
Cong Y, Feng Y, Ni H, et al (2021) Anchor-Locker Binding mechanism of the coronavirus spike protein to human ACE2: insights from computational analysis. J Chem Inf Model 61, 7, 3529–3542. https://doi.org/10.1021/acs.jcim.1c00241
Collantes MEV, Espiritu AI, Sy MCC et al (2021) Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci 48:66–76
Koyuncu O, Hogue I, Enquist L (2013) Virus infections in the nervous system. Cell Host Microbe 13:379–393
Chilvers MA, McKean M, Rutman A et al (2001) The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J 18:965–970
Baig AM, Khaleeq A, Ali U, Syeda H (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11:995–998
Boldrini M, Canoll PD, Klein RS (2021) How COVID-19 affects the brain. JAMA Psychiatry 78:682–683
Swanson PA II, McGavern DB (2015) Viral diseases of the central nervous system. Curr Opin Virol 11:44–54
Meinhardt J, Radke J, Dittmayer C et al (2021) Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 24:168–175
Wu Y, Xu X, Chen Z et al (2020) Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 87:18–22
Poyiadji N, Shahin G, Noujaim D et al (2020) COVID-19–associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology 296(2):E119–E120. https://doi.org/10.1148/radiol.2020201187
Daly JL, Simonetti B, Klein K et al (2020) Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 370:861–865
Al-Dalahmah O, Thakur KT, Nordvig AS et al (2020) Neuronophagia and microglial nodules in a SARS-CoV-2 patient with cerebellar hemorrhage. Acta Neuropathol Commun 8:1–7
Wadhawan A, Stiller JW, Potocki E et al (2019) Traumatic brain injury and suicidal behavior: a review. J Alzheimers Dis 68:1339–1370
Niazkar HR, Zibaee B, Nasimi A, Bahri N (2020) The neurological manifestations of COVID-19: a review article. Neurol Sci 41:1667–1671
Islam MA, Alam SS, Kundu S et al (2020) Prevalence of headache in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of 14,275 patients. Front Neurol 11:562634. https://doi.org/10.3389/fneur.2020.562634
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. JAMA Neurol 395:683–690
Zhao Z, Wei Y, Tao C (2020) An enlightening role for cytokine storm in coronavirus infection. Clin Immunol 222:108615. https://doi.org/10.1016/j.clim.2020.108615
Divani AA, Andalib S, Biller J et al (2020) Central nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep 20:1–20
Antoinette M, Jan DAH (2020) Headache medication and the COVID-19 pandemic. J Headache Pain 21;38. https://doi.org/10.1186/s10194-020-01106-5
Rinott E, Kozer E, Shapira Y et al (2020) Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect 26(9):1259.e5–1259.e7. https://doi.org/10.1016/j.cmi.2020.06.003
Bolay H, Özge A, Uludüz D, Baykan B (2020) Are migraine patients at increased risk for symptomatic coronavirus disease 2019 due to shared comorbidities? Headache J Head Face Pain 60:2508–2521
Zeng M, Wang D-Y, Mullol J, Liu Z (2021) Chemosensory dysfunction in patients with COVID-19: what do we learn from the global outbreak? Curr Allergy Asthma Rep 21:1–8
Boudjema S, Finance J, Coulibaly F et al (2020) Olfactory and gustative disorders for the diagnosis of COVID-19. Travel Med Infect Dis 37:101875
Zubair AS, McAlpine LS, Gardin T et al (2020) Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol 77:1018–1027
Yan CH, Faraji F, Prajapati DP et al (2020) Association of chemosensory dysfunction and Covid‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol 10(7):806–813. https://doi.org/10.1002/alr.22579
Vaira LA, Salzano G, Deiana G, De Riu G (2020) Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope 130(7):1787. https://doi.org/10.1002/lary.28692
Chacko M, Job A, Caston F et al (2020) COVID-19-induced psychosis and suicidal behavior: case Report. SN Compr Clin Med 2:2391–2395
Subbarao K, Roberts A (2006) Is there an ideal animal model for SARS? Trends Microbiol 14:299–303
Ferrando SJ, Klepacz L, Lynch S et al (2020) COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? Psychosomatics 61:551
Fischer M, Coogan AN, Faltraco F, Thome J (2020) COVID-19 paranoia in a patient suffering from schizophrenic psychosis–a case report. Psychiatry Res 288:113001
Severance EG, Dickerson FB, Viscidi RP et al (2011) Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr Bull 37:101–107
Zielinski MR, McKenna JT, McCarley RW (2016) Functions and mechanisms of sleep. AIMS Neurosci 3:67
Alimoradi Z, Broström A, Tsang HWH et al (2021) Sleep problems during COVID-19 pandemic and its’ association to psychological distress: a systematic review and meta-analysis. EClinicalMedicine 36:100916
Yue L, Wang J, Ju M et al (2020) How psychiatrists coordinate treatment for COVID-19: a retrospective study and experience from China. Gen Psychiatry 33(4):e100272. https://doi.org/10.1136/gpsych-2020-100272
Lin YN, Liu ZR, Li SQ et al (2021) Burden of sleep disturbance during COVID-19 pandemic: a systematic review. Nat Sci Sleep 13:933
Moldofsky H, Patcai J (2011) Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol 11:1–7
Nannoni S, de Groot R, Bell S, Markus HS (2021) Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke 16:137–149
Merkler AE, Parikh NS, Mir S et al (2020) Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol 77,11 1–7. https://doi.org/10.1001/jamaneurol.2020.2730
Spence JD, De Freitas GR, Pettigrew LC et al (2020) Mechanisms of stroke in COVID-19. Cerebrovasc Dis 49:451–458
Li Y, Li M, Wang M et al (2020) Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol 5(3):279–284. https://doi.org/10.1136/svn-2020-000431
Hess DC, Eldahshan W, Rutkowski E (2020) COVID-19-related stroke. Transl Stroke Res 11:322–325
Iba T, Levy JH, Warkentin TE et al (2019) Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost 17:1989–1994
Valderrama EV, Humbert K, Lord A et al (2020) Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke 51(7):e124–e127. https://doi.org/10.1161/STROKEAHA.120.030153
Wang H, Tang X, Fan H et al (2020) Potential mechanisms of hemorrhagic stroke in elderly COVID-19 patients. Aging (Albany NY) 12:10022
Ostovan VR, Foroughi R, Rostami M et al (2021) Cerebral venous sinus thrombosis associated with COVID-19: a case series and literature review. J Neurol 268(10):3549–3560. https://doi.org/10.1007/s00415-021-10450-8
Zhang Y, Xiao M, Zhang S et al (2020) Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 382:e38
Beyrouti R, Adams ME, Benjamin L et al (2020) Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry 91:889–891
Hughes C, Nichols T, Pike M et al (2020) Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med 7(5):001691. https://doi.org/10.12890/2020_001691
Moriguchi T, Harii N, Goto J et al (2020) A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 94:55–58
Kansagra SM, Gallentine WB (2011) Cytokine storm of acute necrotizing encephalopathy. Pediatr Neurol 45:400–402
Guilmot A, Slootjes SM, Sellimi A et al (2021) Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol 268:751–757
Wilson JE, Mart MF, Cunningham C et al (2020) Delirium. Nat Rev Dis Prim 6:1–26
Helms J, Kremer S, Merdji H et al (2020) Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 382:2268–2270
Kotfis K, Roberson SW, Wilson JE et al (2020) COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care 24:1–9
El‐Qushayri AE, Ghozy S, Reda A et al (2021) The impact of Parkinson’s disease on manifestations and outcomes of Covid‐19 patients: a systematic review and meta‐analysis. Rev Med Virol 14:e2278. https://doi.org/10.1002/rmv.2278
McLean G, Hindle JV, Guthrie B, Mercer SW (2017) Co-morbidity and polypharmacy in Parkinson’s disease: insights from a large Scottish primary care database. BMC Neurol 17:1–8
Bhidayasiri R, Virameteekul S, Kim J-M et al (2020) COVID-19: an early review of its global impact and considerations for Parkinson’s disease patient care. J Mov Disord 13:105
Li Y, Bai W, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 92:552–555
Rajan S, Kaas B, Moukheiber E (2019) Movement disorders emergencies. Semin Neurol 39(1):125–136. https://doi.org/10.1055/s-0038-1677050
Chan JL, Murphy KA, Sarna JR (2021) Myoclonus and cerebellar ataxia associated with COVID-19: a case report and systematic review. J Neurol 268(10):3517–3548. https://doi.org/10.1007/s00415-021-10458-0
Oh S-Y, Kim J-S, Dieterich M (2019) Update on opsoclonus–myoclonus syndrome in adults. J Neurol 266:1541–1548
Armangué T, Sabater L, Torres-Vega E et al (2016) Clinical and immunological features of opsoclonus-myoclonus syndrome in the era of neuronal cell surface antibodies. JAMA Neurol 73:417–424
Wan S, Yi Q, Fan S et al (2020) Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). MedRxiv https://doi.org/10.1101/2020.02.10.20021832
Chen C, Zhang XR, Ju ZY, He WF (2020) Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi 36(6):471–475. Chinese. https://doi.org/10.3760/cma.j.cn501120-20200224-00088
Marchandot B, Sattler L, Jesel L et al (2020) COVID-19 related coagulopathy: a distinct entity? J Clin Med 9:1651
Goh Y, Beh DLL, Makmur A et al (2020) Pearls and oysters: facial nerve palsy as a neurological manifestation of Covid-19 infection. Neurology 25;95(8):364–367. https://doi.org/10.1212/WNL.0000000000009863
Tamaki A, Cabrera CI, Li S et al (2021) Incidence of Bell palsy in patients with COVID-19. JAMA Otolaryngol Neck Surg 147:767–768
Nepal G, Rehrig JH, Shrestha GS et al (2020) Neurological manifestations of COVID-19: a systematic review. Crit Care 24:1–11
Revzin MV, Raza S, Srivastava NC et al (2020) Multisystem imaging manifestations of COVID-19, part 2: from cardiac complications to pediatric manifestations. Radiographics 40:1866–1892
Caress JB, Castoro RJ, Simmons Z et al (2020) COVID-19–associated Guillain-Barré syndrome: the early pandemic experience. Muscle Nerve 62:485–491
Ramani SL, Samet J, Franz CK et al (2021) Musculoskeletal involvement of COVID-19: review of imaging. Skelet Radiol 50(9):1763–1773. https://doi.org/10.1007/s00256-021-03734-7
Ahlawat S, Chhabra A, Blakely J (2014) Magnetic resonance neurography of peripheral nerve tumors and tumorlike conditions. Neuroimaging Clin 24:171–192
Sedaghat Z, Karimi N (2020) Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci 76:233–235
van Vuren EJ, Steyn SF, Brink CB et al (2021) The neuropsychiatric manifestations of COVID-19: interactions with psychiatric illness and pharmacological treatment. Biomed Pharmacother 135:111200. https://doi.org/10.1016/j.biopha.2020.111200
Roy D, Ghosh R, Dubey S et al (2020) Neurological and neuropsychiatric impacts of COVID-19 pandemic. Can J Neurol Sci 48(1):9–24. https://doi.org/10.1017/cjn.2020.173
Janiri D, Petracca M, Moccia L et al (2020) COVID-19 pandemic and psychiatric symptoms: the impact on Parkinson’s disease in the elderly. Front Psychiatry 11:1306
Vittori A, Lerman J, Cascella M et al (2020) Coronavirus Disease 2019 Pandemic Acute Respiratory Distress Syndrome Survivors: Pain After the Storm? Anesth Analg 31(1):117–119. 10.1213/ANE.0000000000004914
Xie Q, Liu X-B, Xu Y-M, Zhong B-L (2021) Understanding the psychiatric symptoms of COVID-19: a meta-analysis of studies assessing psychiatric symptoms in Chinese patients with and survivors of COVID-19 and SARS by using the Symptom Checklist-90-Revised. Transl Psychiatry 11:1–10
Miller AH, Haroon E, Raison CL, Felger JC (2013) Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30:297–306
Nakamura ZM, Nash RP, Laughon SL, Rosenstein DL (2021) Neuropsychiatric complications of COVID-19. Curr Psychiatry Rep 23:1–9
Stamenkovic DM, Laycock H, Karanikolas M et al (2019) Chronic pain and chronic opioid use after intensive care discharge–is it time to change practice? Front Pharmacol 10:23
Taquet M, Geddes JR, Husain M et al (2021) 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 8:416–427
Rogers JP, Chesney E, Oliver D et al (2020) Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 7:611–627
Garrigues E, Janvier P, Kherabi Y et al (2020) Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 81(6):e4–e6. https://doi.org/10.1016/j.jinf.2020.08.029
Varatharaj A, Thomas N, Ellul MA et al (2020) Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 7:875–882
Liotta EM, Batra A, Clark JR et al (2020) Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol 7:2221–2230
Woo MS, Malsy J, Pöttgen J et al (2020) Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun 2(2):fcaa205. https://doi.org/10.1093/braincomms/fcaa205
Nasreddine ZS, Phillips NA, Bedirian V (2006) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. JAm Geriatr Soc 2005; 53: 695–9. Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges J. The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive. Int J Geriatr Psychiatry 21:1075–1078
Cella D, Riley W, Stone A et al (2010) The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 63:1179–1194
Wang QQ, Kaelber DC, Xu R, Volkow ND (2021) COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry 26:30–39
Tomashefski JF Jr, Felo JA (2004) The pulmonary pathology of illicit drug and substance abuse. Curr Diagn Pathol 10:413–426
Hulin J, Brodie A, Stevens J, Mitchell C (2020) Prevalence of respiratory conditions among people who use illicit opioids: a systematic review. Addiction 115:832–849
Schimmel J, Manini AF (2020) Opioid use disorder and COVID-19: biological plausibility for worsened outcomes. Subst Use Misuse 55:1900–1901
Deng J, Zhou F, Hou W et al (2020) The prevalence of depression, anxiety, and sleep disturbances in COVID‐19 patients: a meta‐analysis. Ann N Y Acad Sci 486(1):90–111. https://doi.org/10.1111/nyas.14506
Parker C, Shalev D, Hsu I et al (2021) Depression, anxiety, and acute stress disorder among patients hospitalized with COVID-19: a prospective cohort study. J Acad Consult Psychiatry 62:211–219
Mazza MG, De Lorenzo R, Conte C et al (2020) Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun 89:594–600
Gillett G, Jordan I (2020) Severe psychiatric disturbance and attempted suicide in a patient with COVID-19 and no psychiatric history. BMJ Case Rep 13:e239191
Khawam E, Khouli H, Pozuelo L (2020) Treating acute anxiety in patients with COVID-19. Cleve Clin J Med. https://doi.org/10.3949/ccjm.87a.ccc016
Han RH, Schmidt MN, Waits WM et al (2020) Planning for mental health needs during COVID-19. Curr Psychiatry Rep 22:1–10
Chamberlain SR, Grant JE, Trender W et al (2021) Post-traumatic stress disorder symptoms in COVID-19 survivors: online population survey. BJPsych Open 9;7(2):e47. https://doi.org/10.1192/bjo.2021.3
Chang MC, Park D (2020) Incidence of post-traumatic stress disorder after coronavirus disease. In: Healthcare. Multidisciplinary Digital Publishing Institute. Healthcare (Basel) 30;8(4):373. https://doi.org/10.3390/healthcare8040373
Halpin SJ, McIvor C, Whyatt G et al (2021) Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 93:1013–1022
Hafeez A, Ahmad S, Siddqui SA et al (2020) A review of COVID-19 (Coronavirus Disease-2019) diagnosis, treatments and prevention. EJMO 4:116–125
(2021) Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. In: FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0. Accessed 24 Jul 2021
Healthcare C, Kft H. MEDICINAL PRODUCT FOR USE □ Name of the medicinal product for Use: Regkirona □ Active substance(s): Regdanvimab □ Pharmaceutical form: Concentrate for solution for infusion □ Route of administration: Intravenous infusion □ Strength: 60 mg/ml 2. NAME AND
Yang Y, Islam MS, Wang J et al (2020) Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci 16:1708
Patel SKS, Lee J-K, Kalia VC (2020) Deploying biomolecules as anti-COVID-19 agents. Indian J Microbiol 60:263–268
Forchette L, Sebastian W, Liu T (2021) A comprehensive review of COVID-19 virology, vaccines, variants, and therapeutics. Curr Med Sci 41(6):1037–1051. https://doi.org/10.1007/s11596-021-2395-1
(2021) COVID-19 vaccine tracker. In: RAPS. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker. Accessed 18 Aug 2021
Jalkanen P, Kolehmainen P, Häkkinen HK et al (2021) (2021) COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun 121(12):1–11. https://doi.org/10.1038/s41467-021-24285-4
Sargent J, Kumar S, Buckley K et al (2021) Positive New Data for Johnson & Johnson Single-Shot COVID-19 Vaccine on Activity Against Delta Variant and Long-lasting Durability of Response
Choi B, Choudhary MC, Regan J et al (2020) Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med 383(23):2291–2293. https://doi.org/10.1056/NEJMc2031364
Sa K, Da C, Rp D et al (2021) SARS-CoV-2 evolution during treatment of chronic infection. Nature 592:277–282. https://doi.org/10.1038/S41586-021-03291-Y
Wang R, Chen J, Gao K, Wei GW (2021) Vaccine-escape and fast-growing mutations in the United Kingdom, the United States, Singapore, Spain, India, and other COVID-19-devastated countries. Genomics 113(4):2158–2170. https://doi.org/10.1016/j.ygeno.2021.05.006
Collier DA, De Marco A, Ferreira IATM et al (2021) Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 593(7857):136–141. https://doi.org/10.1038/s41586-021-03412-7
Madhi SA, Baillie V, Cutland CL et al (2021) Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B. 1.351 variant. N Engl J Med 384:1885–1898
Gupta RK (2021) Will SARS-CoV-2 variants of concern affect the promise of vaccines? Nat Rev Immunol 21:340–341
Jarrahi A, Ahluwalia M, Khodadadi H et al (2020) Neurological consequences of COVID-19: what have we learned and where do we go from here? J Neuroinflamm 17:1–12
Guan W, Ni Z, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720
Wang X, Fang J, Zhu Y et al (2020) Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect 26:1063–1068
Feng Y, Ling Y, Bai T et al (2020) COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med 201:1380–1388
Travi G, Rossotti R, Merli M et al (2021) Neurological manifestations in patients hospitalized with COVID-19: A retrospective analysis from a large cohort in Northern Italy. Eur J Neurosci 53:2912–2922
Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E et al (2020) Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology 95:e1060–e1070
Tian S, Hu N, Lou J et al (2020) Characteristics of COVID-19 infection in Beijing. J Infect 80:401–406
Chen T, Dai Z, Mo P et al (2020) Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol Ser A 75:1788–1795
Du R-H, Liang L-R, Yang C-Q et al (2020) Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J 7;55(5):2000524. https://doi.org/10.1183/13993003.00524-2020
Wang D, Hu B, Hu C et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323:1061–1069
Karadaş Ö, Öztürk B, Sonkaya AR (2020) A prospective clinical study of detailed neurological manifestations in patients with COVID-19. Neurol Sci 41:1991–1995
Wu J, Liu J, Zhao X et al (2020) Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis 71:706–712
Pinna P, Grewal P, Hall JP et al (2020) Neurological manifestations and COVID-19: experiences from a tertiary care center at the frontline. J Neurol Sci 415:116969
Wu J, Wu X, Zeng W et al (2020) Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest Radiol 55(5):257–261. https://doi.org/10.1097/RLI.0000000000000670
Kim ES, Chin BS, Kang CK et al (2020) Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci 6;35(13):e142. https://doi.org/10.3346/jkms.2020.35.e142
Acknowledgements
The authors would like to thank Prof. Prem Kumar Khosla, Vice-Chancellor, Shoolini University, Solan, for providing financial support and necessary facilities.
Author information
Authors and Affiliations
Contributions
Shivam Bhola: Conceptualization; data curation; formal analysis; writing, original draft; writing, review and editing; validation. Jhillika Trisal: Conceptualization, data curation, formal analysis, writing, original draft; writing, review and editing; validation. Vikram Thakur: Data curation; formal analysis; writing, review and editing; validation. Parneet Kaur: Formal analysis, validation. Sourabh Kulshrestha: Data curation, formal analysis, validation. Pradeep Kumar: Conceptualization; formal analysis; writing, review and editing; validation.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhola, S., Trisal, J., Thakur, V. et al. Neurological toll of COVID-19. Neurol Sci 43, 2171–2186 (2022). https://doi.org/10.1007/s10072-022-05875-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-05875-6