Abstract
Introduction
A growing body of evidence that glial cell line–derived neurotrophic factor (GDNF) levels are probably involved in pathogenesis and disease course of Alzheimer’s disease (AD) suggested that its blood levels could potentially be used as a biomarker of AD. The aim of this study was to compare serum GDNF levels in patients with AD and age-matched controls.
Methods
Serum concentrations of GDNF were compared in 25 AD patients and 25 healthy volunteers using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA). Severity of the disease in AD patients was assessed using Functional Assessment Staging (FAST). Cognitive assessment of the patients was done using the Mini-Mental State Examination (MMSE).
Results
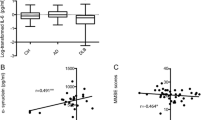
Mean GDNF levels were found to be 2.45 ± 0.93 ng/ml in AD patients and 4.61 ± 3.39 ng/ml in age-matched controls. There was a statistically significant difference in GDNF serum levels in patients with AD compared to age-matched controls (p = 0.001). Moreover, GDNF serum levels were significantly correlated with disease severity (p < 0.001) and cognitive impairment (p < 0.001).
Conclusion
This study showed that serum levels of GDNF are significantly decreased in AD patients in comparison with age-matched controls, thus suggesting a potential role of GDNF as a disease biomarker. However, a comprehensive study of changes in serum levels of multiple neurotrophic factors reflective of different neurobiological pathways in large-scale population studies is recommended.



Similar content being viewed by others
References
Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT (2016) Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 8:23
Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 80:1778–1783
Prince M, Guerchet M, Prina M (2013) Policy brief for heads of government: the global impact of dementia 2013–2050. Alzheimer's Disease International, London https://www.alz.co.uk/research/GlobalImpactDementia2013.pdf. Accessed 20 Sept 2020
Adlimoghaddam A, Roy B, Albensi BC (2018) Future trends and the economic burden of dementia in Manitoba: comparison with the rest of Canada and the world. Neuroepidemiology 51:71–81
Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M, Alzheimer's Disease International (2005) Global prevalence of dementia: a Delphi consensus study. Lancet 366:2112–2117
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E (2011) Alzheimer’s disease. Lancet 377:1019–1031
Krashia P, Nobili A, D'Amelio M (2019) Unifying hypothesis of dopamine neuron loss in neurodegenerative diseases: focusing on Alzheimer’s disease. Front Mol Neurosci 12:123
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298:789–791
Vyas Y, Montgomery JM, Cheyne JE (2020) Hippocampal deficits in amyloid-β-related rodent models of Alzheimer’s disease. Front Neurosci 14:266
Marsh J, Alifragis P (2018) Synaptic dysfunction in Alzheimer’s disease: the effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention. Neural Regen Res 13:616–623
Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wölfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Götz J (2010) Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 142:387–397
Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316:750–754
Angelucci F, Spalletta G, di Iulio F, Ciaramella A, Salani F, Colantoni L, Varsi AE, Gianni W, Sancesario G, Caltagirone C, Bossù P (2010) Alzheimer’s disease (AD) and mild cognitive impairment (MCI) patients are characterized by increased BDNF serum levels. Curr Alzheimer Res 7:15–20
Duan Y, Dong S, Gu F, Hu Y, Zhao Z (2012) Advances in the pathogenesis of Alzheimer’s disease: focusing on tau-mediated neurodegeneration. Transl Neurodegener 1:24
Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S (2008) New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev 59:201–220
Schindowski K, Belarbi K, Buee L (2008) Neurotrophic factors in Alzheimer’s disease: role of axonal transport. Genes Brain Behav 1:43–56
Budni J, Bellettini-Santos T, Mina F, Garcez ML, Zugno AI (2015) The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis 6:331–341
O'Bryant SE, Hobson V, Hall JR, Waring SC, Chan W, Massman P, Lacritz L, Cullum CM, Diaz-Arrastia R, Texas Alzheimer’s Research Consortium (2009) Brain-derived neurotrophic factor levels in Alzheimer’s disease. J Alzheimers Dis 17:337–341
Mattson MP, Maudsley S, Martin B (2004) BDNF and 5-HT: a dynamic duo in age- related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27:589–594
Mitra S, Behbahani H, Eriksdotter M (2019) Innovative therapy for Alzheimer’s disease-with focus on biodelivery of NGF. Front Neurosci 5:13–38
Deister C, Schmidt CE (2006) Optimizing neurotrophic factor combinations for neurite outgrowth. J Neural Eng 3:172–179
Cheng H, Fu YS, Guo JW (2004) Ability of GDNF to diminish free radical production leads to protection against kainate-induced excitotoxicity in hippocampus. Hippocampus 14:77–86
Mätlik K, Võikar V, Vilenius C, Kulesskaya N, Andressoo JO (2018) Two-fold elevation of endogenous GDNF levels in mice improves motor coordination without causing side-effects. Sci Rep 8:11861
Pertusa M, García-Matas S, Mammeri H, Adell A, Rodrigo T, Mallet J, Cristòfol R, Sarkis C, Sanfeliu C (2008) Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging 29:1366–1379
Forlenza OV, Miranda AS, Guimar I, Talib LL, Diniz BS, Gattaz WF, Teixeira AL (2015) Decreased neurotrophic support is associated with cognitive decline in non-demented subjects. J Alzheimers Dis 46:423–429
Airavaara M, Pletnikova O, Doyle ME, Zhang YE, Troncoso JC, Liu QR (2011) Identification of novel GDNF isoforms and cis-antisense GDNFOS gene and their regulation in human middle temporal gyrus of Alzheimer disease. J Biol Chem 286:45093–45102
Ghribi O, Herman MM, Forbes MS, DeWitt DA, Savory J (2001) GDNF protects against aluminum-induced apoptosis in rabbits by upregulating Bcl-2 and Bcl-XL and inhibiting mitochondrial Bax translocation. Neurobiol Dis 8:764–773
Lee JG, Shin BS, You YS, Kim JE, Yoon SW, Jeon DW, Baek JH, Park SW, Kim YH (2009) Decreased serum brain-derived neurotrophic factor levels in elderly Korean with dementia. Psychiatry Investig 6:299–305
Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, Wittorf A, Soekadar S, Richartz E, Koehler N, Bartels M, Buchkremer G, Schott K (2007) BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res 41:387–394
Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, Bartels M, Buchkremer G, Schott K (2006) Stage-dependent BDNF serum concentrations in Alzheimer's disease. J Neural Transm 113:1217–1224
McKhann GM (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269
Sclan SG, Reisberg B (1992) Functional assessment staging (FAST) in Alzheimer’s disease: reliability, validity, and ordinality. Int Psychogeriatr 4:55–69
Reisberg B, Wegiel J, Franssen E, Monteiro I, Torossian C, Anwar S, Gill T, Boksay I, Auer S, Shimada M, Meguro K (2011) The Fast: a brief, practical, comprehensive, valid functional assessment for Alzheimer’s disease staging, diagnosis and differential diagnosis in the primary care setting. Alzheimers Dement 7:S82
Ansari NN, Naghdi S, Hasson S, Valizadeh L, Jalaei (2010) Validation of a mini-mental state examination (MMSE) for the Persian population: a pilot study. Appl Neuropsychol 17:190–195
Gunstad J, Benitez A, Smith J, Glickman E, Spitznagel MB, Alexander T, Juvancic-Heltzel J, Murray L (2008) Serum brain-derived neurotrophic factor is associated with cognitive function in healthy older adults. J Geriatr Psychiatry Neurol 21:166–170
Fielder GC, Wen-Shan Yang T, Razdan M, Li Y, Lu J, Perry JK, Lobie PE, Liu DX (2018) The GDNF family: a role in cancer? Neoplasia 20:99–117
Straten G, Eschweile GW, Maetzler W, Laske C, Leyhe T (2009) Glial cell-line derived neurotrophic factor (GDNF) concentrations in cerebrospinal fluid and serum of patients with early Alzheimer’s disease and normal controls. J Alzheimers Dis 18:331–337
Konishi Y, Yang LB, He P, Lindholm K, Lu B, Li R, Shen Y (2014) Deficiency of GDNF receptor GFRα1 in Alzheimer’s neurons results in neuronal death. J Neurosci 34:13127–13138
Faria MC, Goncalves GS, Rocha NP, Moraes EN, Bicalho MA, Gualberto MT, Jardim de Paula J, José Ravic de Miranda LF, Clayton de Souza Ferreira A, Teixeira AL, Gomes KB, Carvalho M, Sousa LP (2014) Increased plasma levels of BDNF and inflammatory markers in Alzheimer’s disease. J Psychiatr Res 53:166–172
Borba EM, Duarte JA, Bristot G, Scotton E, Camozzato AL, Fagundes Chaves ML (2016) Brain-derived neurotrophic factor serum levels and hippocampal volume in mild cognitive impairment and dementia due to Alzheimer disease. Dement Geriatr Cogn Dis Extra 6:559–567
Leyhe T, Stransky E, Eschweiler GW, Buchkremer G, Laske C (2008) Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 258:124–128
Gezen-Ak D, Dursun E, Hanağasi H, Bilgiç B, Lohman E, Araz OS, Atasoy IL, Alaylıoğlu M, Önal B, Gürvit H, Yılmazer S (2013) BDNF, TNFα, HSP90, CFH, and IL- 10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. J Alzheimers Dis 37:185–195
Janel N, Alexopoulos P, Badel A, Lamari F, Camproux AC, Lagarde J, Simon S, Feraudet-Tarisse C, Lamourette P, Arbones M, Paul JL, Dubois B, Potier MC, Sarazin M, Delabar JM (2017) Combined assessment of DYRK1A, BDNF and homocysteine levels as diagnostic marker for Alzheimer’s disease. Transl Psychiatry 7:e1154
Woolley JD, Strobl EV, Shelly WB, Karydas AM, Robin Ketelle RN, Wolkowitz OM, Miller BL, Rankin KP (2012) BDNF serum concentrations show no relationship with diagnostic group or medication status in neurodegenerative disease. Curr Alzheimer Res 9:815–821
Sonali N, Tripathi M, Sagar R, Vivekanandhan S (2013) Val66Met polymorphism and BDNF levels in Alzheimer’s disease patients in north Indian population. Int J Neurosci 123:409–416
Ted NG, Ho CS, Tam W, Kua EH, Ho RC (2016) Serum brain-derived neurotrophic factors (BDNF) levels in patients with Alzheimer’s disease (AD), individuals with mild cognitive impairment (MCI) and healthy controls: a systematic review, meta-analysis, and meta-regression. Alzheimers Dement 12:1181
Balietti M, Giuli C, Fattoretti P, Fabbietti P, Papa R, Postacchini D, Conti F (2017) Effect of a comprehensive intervention on plasma BDNF in patients with Alzheimer’s disease. J Alzheimers Dis 57:7–43
Konukoglu D, Andican G, Fırtına S, Erkol G, Kurt A (2012) Serum brain-derived neurotrophic factor, nerve growth factor and neurotrophin-3 levels in dementia. Acta Neurol Belg 112:255–260
Pláteník J, Fišar Z, Buchal R, Jirák R, Kitzlerová E, Zvěřová M, Raboch J (2014) GSK3β, CREB, and BDNF in peripheral blood of patients with Alzheimer’s disease and depression. Prog Neuro-Psychopharmacol Biol Psychiatry 3:83–93
Ziegenhorn AA, Schulte-Herbrüggen O, Danker-Hopfe H, Malbranc M, Hartung HD, Anders D, Lang UE, Steinhagen-Thiessen E, Schaub RT, Hellweg R (2007) Serum neurotrophins--a study on the time course and influencing factors in a large old age sample. Neurobiol Aging 28:1436–1445
Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA (2008) Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry 65:439–445
Acknowledgments
The authors would like to express their gratitude to all personnel of Yaadmaan Referral Center for Dementia and Cognitive Disorders, Malard’s Diagnostic Laboratory, and all the patients, who participated in our study. We are under no doubt that this study would not be completed without them. Moreover, the authors wish to thank Dr. Elnaz Roohi for her insightful comments in editing and reviewing of the manuscript. Academic research fund was provided by Tehran Medical Sciences, IAU. It is important to note that Tehran Medical Sciences, IAU, is a non-profit non-governmental organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Tehran Medical Sciences, IAU) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed written consents were obtained from all the participants or their legally authorized representatives prior to the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dr. Maryam Sharif and Dr. Maryam Noroozian share first authorship.
Rights and permissions
About this article
Cite this article
Sharif, M., Noroozian, M. & Hashemian, F. Do serum GDNF levels correlate with severity of Alzheimer’s disease?. Neurol Sci 42, 2865–2872 (2021). https://doi.org/10.1007/s10072-020-04909-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04909-1



