Abstract
Irradiation injury, especially caused by UVB, of the skin is one of the critical reasons for skin inflammation and damage. The present study aimed to explore the protective effect of Syzygium formosum leafy extract (SFLE) and its mechanism of action against UVB-induced damages of human keratinocytes. In this study, SFLE was prepared from 100 kg dried leaves using industrial-scale processes. We found that SFLE markedly reduced markers of the skin inflammation in UVB-induced pro-inflammatory cytokines. Only 2 μg/mL of SFLE exhibited significantly stronger anti-inflammatory effects than the fivefold concentration of positive control. Intriguingly, an anti-inflammatory enzyme, heme oxygenase-1 expression was significantly induced by SFLE treatment. MMP-3 and -9 were, but not MMP-1, significantly reduced. SFLE inhibited the expression of the MAPK pathway, resulting in a decrease on UVB-induced reactive oxygen species. In conclusion, SFLE can potentially be used to treat skin inflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin aging is a result of different factors, such as exposure to ultraviolet (UV) radiation, as well as physical and chemical agents (Parrado et al., 2019; Park et al., 2021). UVB radiation is known to cause various adverse effects on the skin, including but not limited to edema, erythema, hyperplasia, immunosuppression, inflammation, sunburn, skin photoaging, and photocarcinogenesis (Aquino et al., 2002). Among them, photoaging accounts for about 80% of skin aging (Kammeyer and Luiten, 2015).
The UV spectrum is divided into three distinct wavelengths, namely UVA (320–400 nm), UVB (290–320 nm) and UVC (200–290 nm). While UVC is absorbed into the ozone layer, UVA and UVB can reach the Earth’s surface (Cela et al, 2015). UVB irradiation is known to induce the production of inflammatory cytokines (Bashir et al., 2009; Ishida and Sakaguchi, 2007). As the results of UVB irradiation, erythema, edema and epidermal hyperplasia are often found on the skin (De Fabo and Noonan, 1983; El Ghiss et al., 2009). The role of keratinocytes in various responses to UVB-induced photo-damage is well established. (Bashir et al., 2009; Ishida and Sakaguchi, 2007). Therefore, treatment modalities that reduce or block the generation of inflammatory cytokines are important to prevent inflammatory diseases of the skin. When the skin is exposed to UVB irradiation, the inflammatory response to the degenerative process is mediated primarily by the overproduction of active oxygen species (ROS) in keratinocytes. ROS induces inflammation responses and serious DNA damages (Hanson and Clegg, 2002).
In particular, the activation of mitogen-activated protein kinases (MAPK) is critical for the generation of inflammatory cytokines by UVB irradiation ( Davis, 1993; Fisher et al., 1998; Karin and Hunter, 1995). The MAPK has three family members that include extracellular-signal-related protein (ERK), c-JUN-N-terminal kinases (JNK) and p38 MAPK (Kallunki et al., 1994). The MAPK signaling pathway participates in numerous cellular processes such as cell proliferation, development, cell cycle, morphogenesis, stress resistance, and intercellular signaling (Juretic et al., 2001; Yosimichi et al., 2001). This signaling pathway in turn activates a variety of extracellular matrix proteins including metrix metalloproteninases (MMP) -1, -3 and -9, which are known to be associated with skin senescence, breakdown of collagen and are thus related to aggravation of photoaging (Lee, 2021).
Syzygium formosum is an evergreen tree in the family Myrtaceae, extensively distributed in Southeast Asia, Brazil, Taiwan and India. S. formosum leaf has been traditionally used as food or tea to treat many inflammatory and immune disorder diseases. Its leafy extract (SFLE) has pharmacological activity such as anti-allergic, anti-bacterial activity, anti-inflammatory and antioxidant activity (Minh, 1968; Nong, 2009; Nguyen et al., 2018; Lee et al., 2006; Chang et al., 1999). Previous studies have reported that SFLE contained greater amount of biologically active phytochemicals than the well-known medicinal plant, Centella aciatica. Triterpenic acids were found as major bio-functional chemicals in SFLE (Park et al., 2021). Despite of the medicinal applications of the plant, only few studies on the anti-inflammation effects of SFLE have been reported. Furthermore, the mechanism of its anti-inflammatory actions has not yet been fully investigated.
In this study, we investigated the possible anti-inflammatory properties of SFLE on inflammatory cytokines in human skin keratinocytes that were induced by UVB radiation. In addition, to explore the potential mechanisms underlying the pharmacological effects of SFLE, we assessed the response of the MAPK signaling pathway and inflammatory regulators. The aim of this study was to explore how SFLE functions by assessing the levels of inflammatory cytokines, ROS, MAPK, HO-1 and MMPs in human skin keratinocytes.
Materials and methods
Preparation of SFLE and C. asiatica extract
A significant amount of S. formosum leaves (more than 100 kg of dry leaves) were collected from the suburbs of Hanoi, Vietnam. Additionally, 1 kg of dried C. asiatica was purchased from Jung Woo-dang (Seoul, Korea). Extraction of both plant materials was carried out using 70% ethanol at a ratio of 12-fold (v/w). The extraction process was performed under specific conditions as previously described [24]. The resulting extracts were analyzed for major triterpenic acids using liquid chromatography-mass spectrometry, following the protocol described by Park et al. (2021).
Cell culture
The HaCaT cells, which are human skin keratinocytes were provided by the Korea Research Institute of Bioscience & Biotechnology (KRIBB, Daejeon, Republic of Korea). The cells were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS, WELGENE, Gyeongsan, Republic of Korea), streptomycin (100 μg/mL) and penicillin (100 units/mL) at 37 °C in a 5% CO2 environment.
Cell viability assay
The HaCaT cells (1 × 105 cells/mL) were seeded on 96-well plate and cultured overnight. SFLE was diluted with indicated concentration with medium and added in cell culture plate. The samples were incubated for 24 h, and then treated with an MTT solution according to standard procedures. The incubation continued for an additional hour before further analysis. After removing the supernatant, 0.1 mL of DMSO was added, and the optical density at 620 nm was measured using a microplate reader. (Epoch, Biotek, Winooski, VT, USA).
Compound treatment and UVB irradiation
To perform the experiment, HaCaT cells were seeded at a concentration of 2 × 105 cells/mL in 60 mm dish and incubated overnight. The cells were then treated with different concentrations of SFLE (2 and 5 μg/mL) and 10 μg/mL of C. asiatica extract as a positive control for 4 h. Subsequently, the cells were washed twice with 2 mL of Dulbecco’s Phosphate-Buffered Saline (DPBS, WELGENE, Gyeongbuk, Republic of Korea) and exposed to 10 mJ/cm2 of UVB. The cells were further incubated in serum-free medium (DMEM) containing the compound for 2 or 4 h.
Reverse transcription polymerase chain reaction (RT-PCR)
The Trizol method was used to extract total RNA from HaCaT cells as per the manufacturer’s instructions (Invitrogen, Waltham, MA, USA). The extracted RNA was then subjected to reverse transcription using AccuPower® CycleScript™ RT PreMix (Bioneer, Daejeon, Republic of Korea) to produce cDNA. To assess the mRNA expression levels of the target genes, PCR analysis was performed. The results were normalized to the expression levels of the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer sequences utilized in this analysis can be found in Supplementary Table 2.
Enzyme-linked immunosorbent assay (ELISA)
To determine the concentrations of inflammatory cytokines, the supernatants of HaCaT cells were collected and analyzed using an ELISA kit (BioLegend, San Diego, CA, USA) according to the manufacturer’s instructions. The cytokines measured included IL-6, IL-8 and TNF-α.
Western blotting
Protein extraction from cultured HaCaT cells was performed using a protease inhibitor cocktail and cell lysis buffer (Cell Signaling Technology, MA, USA). The resulting homogenate was obtained after ultrasonic treatment and centrifugation at 10,000 rpm for 15 min at 4 °C. The sample was separated on a 12% SDS-polyacrylamide gel, and then transferred onto a PVDF membrane that had been blocked with 5% w/v fat-free dry milk in PBS containing 0.5% Tween-20. Primary antibodies, including actin (purchased from Santa Cruz Biotechnology, Dallas, USA), p-ERK (44-680G, ThermoFisher Scientific, MA, USA), ERK, p-JNK, JNK, p-p38, and p38 (all from ThermoFisher Scientific) were used to detect the target proteins.
Measurement of ERK and JNK
To measure ROS levels in HaCaT cells, the cells were first homogenized in cell lysis buffer and then centrifuged at 10,000 rpm for 15 min at 4 °C. The resulting supernatant was used for the quantification of ROS levels using the DCF ROS/RNS Assay kit (Abcam, #238,535, Cambridge, UK), which employs the fluorogenic probe DCFHDiOxyQ that is specific for ROS/RNS. The assay was carried out according to the manufacturer’s instructions as described previously by Vandierendonck et al. (2021). In order to measure the levels of ROS/RNS in the sample, fluorescence intensity was detected at an excitation wavelength of 480 nm and an emission wavelength of 530 nm.
Statistical analysis
The results are presented as mean ± standard error (SEM). Statistical analysis was performed using GraphPad Prism v.5.10 software (GraphPad Software Inc., San Diego, CA, USA), utilizing one-way analysis of variance (ANOVA) followed by post-hoc multiple comparison tests with Tukey’s HSD test. A p-value less than 0.05 was considered statistically significant, and all statistical comparisons between different treatments were evaluated using one-way ANOVA with the Tukey multiple comparison post-test.
Results and discussion
Effects of SFLE and UVB on the viability
Major triterpenes in the extracts are shown in Supplementary Table 1. Asiatic acid, corosolic acid and betulinic acids were major components in SFLE, but only asiatic acid and madecassic acid were detected in C. asiatica extract. Total triterpenic acids were tenfold higher in SFLE than C. asiatica. To identify the effect of SFLE on human skin keratinocytes cells, the cells were cultured with SFLE (0–100 μg/mL) and UVB (0–30 mJ/cm2) for 24 h, and cell viability was measured by MTT assay. SFLE and UVB were not cytotoxic to human skin keratinocyte cells at the concentrations used 2 and 5 μg/mL and 10 mJ/cm2, respectively (Fig. 1).
Effects of SFLE extract and UVB dose on the viability of HaCaT. (A) The cells were treated with indicated concentration (6.25–100 μg/mL) of SFLE for 24 h. (B) The cells were irradiated with various dose of UVB (10–30 mJ/cm2) then incubated in serum-free medium for 4 h. Cell viability was assessed by MTT assay. These results are expressed as the mean ± SEM of three independent experiment
Inhibitory effects of SFLE on UVB-induced inflammation
To identify the inhibitory effects of SFLE on UVB-induced cytokines such as IL-1β, -6, -8, TNF-α and COX-2 in HaCaT keratinocytes, we measured pro-inflammatory cytokines levels using an ELSIA assay. Treatment with UVB significantly increased IL-1β, -6, -8, TNF-α and COX-2 levels compared with the control group, while treatment with 5 μg/mL SFLE decreased UVB-induced IL-1β, -6, -8, TNF-α and COX-2 (Fig. 2A, B). We next examined the effects in the critical inflammatory cytokine secretion such as IL-6, -8 and TNF-α on UVB treatment human skin keratinocytes. UVB-induced IL-6, -8 and TNF-α secretion were blocked by SFLE treatment in human skin keratinocytes (Fig. 2C). These results showed that SFLE treatment suppressed pro-inflammatory cytokines levels.
UVB radiation can have various effects on human health, with one of the most notable being the stimulation of vitamin D production in the skin. Vitamin D plays a crucial role in many physiological processes, including cell proliferation, bone growth, immune function and insulin secretion. A deficiency in vitamin D can lead to various health problems. Therefore, regular and minimum exposure to UVB is necessary to maintain optimal health (Holick, 2003). Despite its importance in stimulating vitamin D production, UVB radiation can also have numerous negative effects on the skin. Acute exposure to UVB can result in inflammation, sunburn, edema, erythema and immunosuppression. These biological effects of UVB on the skin can have significant impacts on human health (Afaq et al., 2005). Inflammatory infiltrating cells irradiated with UVB produce cytokines. In keratinocytes, function and regulation of cytokines have been extensively studied. The IL-6, IL-8 and TNF-α cytokines are known to be associated with the progression of UVB irradiation and are produced by keratinocytes. They have been linked to cellular damage and the development of inflammatory diseases. Studies suggest that SFLE may have anti-inflammatory properties by inhibiting these cytokines. Therefore, SFLE has the potential to be used as a treatment for skin inflammation (Ding et al., 2009; Kovaríková et al., 2004). These results suggest that SFLE has the ability to inhibit pro-inflammatory cytokines, which could make it a promising treatment option for skin inflammation. Overall, this study provides further evidence supporting the potential of SFLE as an anti-inflammatory agent for skin-related conditions.
Inhibitory effects of SFLE on UVB-induced ROS
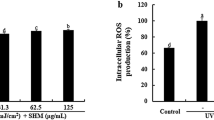
To investigate the effects of SFLE on anti-oxidative enzymes in human skin keratinocytes, we measured the levels of heme oxygenase-1 (HO-1) in cells treated with SFLE and UVB. Our results showed that treatment with UVB significantly decreased HO-1 enzyme levels compared to control cells, but treatment with 5 μg/mL SFLE increased the levels of HO-1 that were reduced by UVB exposure (Fig. 3A, B). Additionally, we assessed the generation of reactive oxygen species using DCFH-DiOxyQ to determine whether SFLE treatment affected ROS levels. Our findings showed that treatment with UVB significantly increased ROS generation compared to control cells, while treatment with 5 μg/mL SFLE decreased UVB-induced ROS generation (Fig. 3c). These results suggest that SFLE treatment can suppress ROS generation in human skin keratinocytes.
Repression of major inflammatory cytokines such as (A) IL-6, (B) IL-8 and (C) TNF-α in UVB-induced HaCaT cells by treating the plant extracts. SFLE were pretreated to the cells 4-h before UVB irradiation. CA indicates Centella asiatica. Statistical significance: *P < 0.05; **P < 0.01; ***P < 0.001 compared with the UVB irradiation group
Inhibition effects of SFLE on UVB-induced matrix metalloproteinase
In order to investigate the potential inhibitory effects of SFLE on MMPs in UVB-damaged human skin keratinocytes, we utilized RT-PCR to analyze the RNA levels of MMP-1, -3 and -9. SFLE decreased the MMP-3 and MMP-9 levels in human skin keratinocytes but did not decrease the MMP-1 levels (Fig. 4). These results showed that SFLE treatment decreased MMP-3 and -9 in human skin keratinocytes. One of the key characteristics of skin that has been affected by photo-aging is the disruption of the connective tissue and subsequent loss of structural integrity in the extracellular matrix. A crucial characteristic of photoaged skin is the impairment of the connective tissue and the loss of structural integrity in its extracellular matrix (ECM) (Yang et al., 2019). In order to maintain optimal hydration and elasticity in the skin, the ECM plays a crucial role in providing a network structure that contains essential structural proteins such as collagen and elastin (Birkedal-Hansen, 1987; Freedberg et al., 2001). MMP-1, a member of the MMP family, not only activates MMP-3 and degrades collagen type IV, but also degrades collagen fragments via MMP-9 (Kwon et al., 2019). In photoaging skin, changes to the composition of the ECM may occur due to high expression of MMPs (Anggakusuma and Hwang, 2010). Signaling molecules such as ERK, JNK and p38 MAPK play an essential role in transmitting extracellular signals into the nucleus to regulate gene expression (Yang et al., 2009). The family of MMPs, secreted by fibroblasts and keratinocytes, includes MMP-1, MMP-3 and MMP-9, and they play a critical role in the process of skin photoaging. Thus, high expression of MMP in photoaged skin may be a major cause of decreased skin elasticity and wrinkle formation. Our study found that treatment with SFLE significantly inhibited MMP-3 and MMP-9, suggesting that SFLE could potentially be used as a therapeutic option to improve the skin’s extracellular matrix.
Anti-oxidative effect SFLE. Cells were pretreated with SFLE 4-h before UVB irradiation. Heme oxygenase-1 (HO-1) expression was measured (A and B) with the Quantification of ROS using DCFH-DiOxyQ (C). Statistical significance: *P < 0.05; **P < 0.01; ***P < 0.001 compared with the UVB irradiation group
Inhibition effects of SFLE on UVB-induced MAPK pathway
MAPK is a crucial signaling pathway involved in the regulation of transcriptional induction of inflammatory molecules. Therefore, the anti-inflammatory pathway suppressed against UVB irradiation by inhibiting MAPK activation. To evaluate the inhibitory effects of SFLE on UVB-induced MAPK signaling in HaCaT keratinocytes, we measured phosphorylation (p-) MAPK (ERK, JNK) using western blot and ELSIA analysis. Compared to control cells, exposure to UVB radiation resulted in a noteworthy elevation of phosphorylated ERK and JNK levels. However, treatment with 5 μg/mL SFLE was found to decrease the UVB-induced levels of phosphorylated MAPK ERK and JNK. This indicates the potential of SFLE to mitigate the effects of UVB-induced skin damage (Fig. 5). These results showed that SFLE treatment suppressed MAPK pathways.
Skin protective mechanism of SFLE against UVB-induced damages
Our investigation represents the first attempt to elucidate the underlying inhibitory mechanism of SFLE on UVB-induced skin inflammation in human keratinocytes, offering novel insights into the potential therapeutic advantages of SFLE in the management of UVB-induced skin damage. We observed that SFLE effectively suppressed UVB-induced skin inflammation by downregulating the expression of pro-inflammatory cytokines (IL-1β, -6, -8 and TNF-α) via its modulation of MAPK signaling pathways. This provides important insights into the potential therapeutic applications of SFLE for treating UVB-induced skin damage.
MAPK regulate important inflammation pathways following stimulation with UVB irradiation (Carlsen et al., 2004; Hong et al., 2009; Muthusamy and Piva, 2010). UVB radiation exposure can lead to the generation of ROS, which activate various cellular signaling pathways, including the MAPK signaling pathways. These pathways, including JNK, ERK and p38, play a critical role in the cellular response to UVB-induced ROS generation, which can mediate skin inflammatory responses and apoptosis (Leng et al., 2009; Neves et al., 2009). In epidermal keratinocytes, various extracellular stimuli, including UVB, can cause stress-related signal cascades, of which the most important is activation of MAPK. Given the ability of SFLE to scavenge ROS and inhibit inflammation, it is reasonable to speculate that many of the biological events triggered by UVB radiation may be mediated by SFLE. These findings highlight the potential of SFLE as a therapeutic agent for preventing or treating skin damage induced by UVB exposure. Among the signaling pathways stimulated by UVB, MAPKs is playing a key role by modulating UVB damaging effects. This study investigated the inhibitory effects of SFLE on UVB-induced MAPK activation, and confirmed its anti-inflammatory properties through its effects on MAPK signaling pathways. These findings shed light on the potential therapeutic applications of SFLE for preventing or treating UVB-induced skin damage. To investigate the potential impact of SFLE on the skin’s oxidative defense system, we assessed levels of the antioxidant enzyme HO-1. This enzyme is known to help repair skin damage by reducing the effects of free radicals, which are common contributors to photoaging. Additionally, we observed a significant decrease in the UVB-induced phosphorylation of ERK and JNK following treatment with SFLE. These findings suggest that SFLE may have potential as a therapeutic agent for preventing and treating skin damage caused by oxidative stress. HO-1 plays a critical role in the catabolism of heme, and its activation leads to the release of iron, biliverdin, and carbon monoxide. These findings provide valuable insights into the potential mechanisms by which SFLE may exert therapeutic effects against UVB-induced skin damage (Park et al., 2012). This study found that UVB exposure resulted in a significant decrease in HO-1 levels, while treatment with SFLE increased the levels of HO-1. Also, we investigated the impact of SFLE on UVB-induced ROS generation by measuring DCFH-DiOxyQ fluorescence in human skin keratinocytes in our study. Treatment with UVB significantly increased ROS generation compared with the control group, while treatment with 5 μg/mL SFLE decreased UVB-induced ROS generation. These findings suggest that SFLE suppresses the inflammation cytokines by inhibiting the MAPK signaling pathway, inhibit the ROS generation via oxidative defense protein. To our knowledge, this is the first study to examine the effects of SFLE on human skin keratinocytes, particularly regarding ROS production, anti-oxidant enzyme reduction, inflammatory cytokines, and matrix metalloproteinase-3 and -9, via the MAPK signaling pathways (Fig. 6). The SFLE has anti-inflammatory effect and be useful as therapeutic agent to relieve photoaging.
We found that SFLE treatment on UVB-irradiated human skin keratinocytes significantly attenuated the production of UVB-stimulated pro-inflammatory cytokines such as IL-1β, -6, -8, COX-2 and TNF-α. It is noteworthy that fivefold lower dose of SFLE (2 μg/mL) exhibited much stronger anti-inflammatory effects than C. asiatica extract. These inhibitory effects were exerted by the inhibition of inflammation-regulated ERK and JNK MAPK pathway signaling. We also found that SFLE inhibited the important matrix metalloproteinase in skin, MMP-3 and MMP-9. In addition, the inhibitory effects of SFLE on UVB-induced inflammation related molecules are associated with decrease of ROS and increase of HO-1. In conclusion, our results indicate that S. formosum leafy extract could be a feasible therapeutic ingredient to attenuate skin senescence via affecting inflammatory cytokines, ROS, MAPK, HO-1 and MMPs in human skin keratinocytes.
References
Afaq F, Adhami VM, Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutation Research. 571: 153–173 (2005)
Anggakusuma, Yanti, Hwang JK. Effects of macelignan isolated from Myristica fragrans Houtt. on UVB-induced matrix metalloproteinase-9 and cyclooxygenase-2 in HaCaT cells. Journal of Dermatological Science. 57: 114–122 (2010)
Aquino R, Morelli S, Tomaino A, Pellegrino M, Saija A, Grumetto L, Puglia C, Ventura D, Bonina F. Antioxidant and photoprotective activity of a crude extract of culcitium reflexum H.B.K. Leaves and their major flavonoids. Journal of Ethnopharmacology. 79: 183–191 (2002)
Bashir MM, Sharma MR, Werth VP. UVB and proinflammatory cytokines synergistically activate TNF-alpha production in keratinocytes through enhanced gene transcription. Journal of Investigative Dermatology. 129: 994–1001 (2009)
Birkedal-Hansen H. Catabolism and turnover of collagens: collagenases. Methods in Enzymology. 144: 140–171 (1987)
Carlsen H, Alexander G, Austenaa LM, Ebihara K, Blomhoff R. Molecular imaging of the transcription factor NF-kappaB, a primary regulator of stress response. Mutation Research. 551: 199–211 (2004)
Cela, EM, Friedrich A, Paz, ML, Vanzulli SI, Leoni J, Gonzalez Maglio, DH. Time-course study of different innate immune mediators produced by UV-irradiated skin: Comparative effects of short and daily versus a single harmful UV exposure. Immunology 145: 82–93 (2015)
Chang CW, Wu TS, Hsieh YS, Kuo SC, Chao PDL. Terpenoids of Syzygium formosanum. Journal of Natural Products. 62: 327–328 (1999)
Davis RJ. The mitogen-activated protein kinase signal transduction pathway. Journal of Biological Chemistry. 268: 14553–14556 (1993)
De Fabo EC, Noonan FP. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. Journal of Experimental Medicine. 158: 84–98 (1983)
Ding C, Cicuttini F, Li J, Jones G. Targeting IL-6 in the treatment of inflammatory and autoimmune diseases. Expert Opinion on Investigational Drugs. 18: 1457–1466 (2009)
El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--part D: radiation. Lancet Oncology. 10: 751–752 (2009)
Fisher GJ, Talwar HS, Lin J, Lin P, McPhillips F, Wang Z, Li X, Wan Y, Kang S, Voorhees JJ. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. Journal of Clinical Investigation. 101: 1432–1440 (1998)
Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. Journal of Investigative Dermatology. 116: 633–640 (2001)
Hanson KM, Clegg RM. Observation and quantification of ultraviolet-induced reactive oxygen species in ex vivo human skin. Photochemistry and Photobiology. 76: 57–63 (2002)
Holick MF. Vitamin D: A millenium perspective. Journal of Cellular Biochemistry. 88: 296–307 (2003)
Hong L, Xiaoqun L, Li Ma, Kefei K, Zhizhong Z. Reversal of ultraviolet B-induced immunosuppression by inhibition of the extracellular signal-regulated mitogen-activated protein kinase. Photodermatol Photoimmunol Photomed. 25: 264–269 (2009)
Ishida T, Sakaguchi I. Protection of human keratinocytes from UVB-induced inflammation using root extract of Lithospermum erythrorhizon. Biological and Pharmaceutical Bulletin. 30: 928–934 (2007)
Juretic N, Santibáñez JF, Hurtado C, Martínez J. ERK 1,2 and p38 pathways are involved in the proliferative stimuli mediated by urokinase in osteoblastic SaOS-2 cell line. Journal of Cellular Biochemistry. 83: 92–98 (2001)
Kallunki T, Su B, Tsigelny I.; Sluss HK, Dérijard B, Moore G, Davis R, Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes & Development. 8: 2996–3007 (1994)
Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Research Reviews. 21: 16–29 (2015)
Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Current Biology. 5: 747–757 (1995)
Kovaríková M, Hofmanová J, Soucek K, Kozubík A. The effects of TNF-alpha and inhibitors of arachidonic acid metabolism on human colon HT-29 cells depend on differentiation status. Differentiation. 72: 23–31 (2004)
Kwon KR, Alam MB, Park JH, Kim TH, Lee SH. Attenuation of UVB-Induced Photo-Aging by Polyphenolic-Rich Spatholobus Suberectus Stem Extract Via Modulation of of MAPK/AP-1/MMPs Signaling in Human Keratinocytes. 384 Nutrients. 11:1341 (2019)
Minh ND. Study on antibiotic activities of Syzygium formosum. Pharm. J. (Vietnam). 14–15 (1968)
Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Archives of Dermatological Research. 302: 5–17 (2010)
Neves BM, Cruz MT, Francisco V, Garcia-Rodriguez C, Silvestre R, Cordeiro-da-Silva A, Dinis AM, Batista MT, Duarte CB, Lopes MC. Differential roles of PI3-Kinase, MAPKs and NF-kappaB on the manipulation of dendritic cell T(h)1/T(h)2 cytokine/chemokine polarizing profile. Molecular Immunology. 46: 2481–2492 (2009)
Nguyen DT, Nguyen TT, Bui TN, Phuong TT. Anti-oxidant activity of Syzygium formosum (Wall.) Masam and the major isolated component. Pharm. J. (Vietnam). 6: 52 (2012)
Nguyen TMN, Lomunova M, Vu TPD, Le BV, Kim YH, Kang JS, Hwang I. Anti-allergic effects of the ethanol extract of Syzygium formosum (Wall.) Masam leaves and its immunoregulatory mechanisms. Journal of Ethnopharmacology. 211: 171–179 (2018)
Nong TL. Research on phytochemical constituents of Syzygium formosum in Thai Nguyen-Vietnam, chemistry department. Thai Ngu. Univer. Edu. (2009)
Park G, Jang DS, Oh MS. Juglans mandshurica leaf extract protects skin fibroblasts from damage by regulating the oxidative defense system. Biochemical and Biophysical Research Communications. 421: 343–348 (2012)
Park C, Park J, Kim WJ, Kim W, Cheong H, Kim SJ. Malonic Acid Isolated from Pinus densiflora Inhibits UVB-Induced Oxidative Stress and Inflammation in HaCaT Keratinocytes. Polymers (Basel). 13: 816 (2021)
Park HA, Kim MY, Lee NY, Lim J, Park KB, Lee CK, Nguyen VD, Kim J, Park JT, Park JI. Variation of Triterpenic Acids in 12 Wild Syzygium formosum and Anti-Inflammation Activity on Human Keratinocyte HaCaT. Plants (Basel). 10: 2428 (2021)
Parrado C, Mercado-Saenz S, Perez-Davo A, Gilaberte Y, Gonzalez S, Juarranz A. Environmental stressors on skin aging. Mechanistic insights. Frontiers in Pharmacology. 10: 759 (2019)
Vandierendonck A, Degroote H, Vanderborght B, Verhelst X, Geerts A, Devisscher L, Van Vlierberghe H. NOX1 inhibition attenuates the development of a pro-tumorigenic environment in experimental hepatocellular carcinoma. Journal of Experimental & Clinical Cancer Research. 40: 40 (2021)
Yang B, Ji C, Kang J, Chen W, Bi Z, Wan Y. Trans-Zeatin inhibits UVB-induced matrix metalloproteinase-1 expression via MAP kinase signaling in human skin fibroblasts. International Journal of Molecular Medicine. 23: 555–560 (2009)
Yang JE, Ngo HTT, Hwang E, Seo SA, Park SW, Yi TH. Dietary enzyme-treated Hibiscus syriacus L. protects skin against chronic UVB-induced photoaging via enhancement of skin hydration and collagen synthesis. Archives of Biochemistry and Biophysics. 662: 190–200 (2019)
Yosimichi G, Nakanishi T, Nishida T, Hattori T, Takano-Yamamoto T, Takigawa M. CTGF/Hcs24 induces chondrocyte differentiation through a p38 mitogen-activated protein kinase (p38MAPK), and proliferation through a p44/42 MAPK/extracellular-signal regulated kinase (ERK). European Journal of Biochemistry. 268: 6058–6065 (2001)
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (No. HP20C0211), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (No. 2022R1A4A103301), and the research fund of Chungnam National University (No. 2021-0696-01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, S.H., Lee, NY., Choi, SH. et al. Molecular mechanism of the anti-inflammatory and skin protective effects of Syzygium formosum in human skin keratinocytes. Food Sci Biotechnol 33, 689–697 (2024). https://doi.org/10.1007/s10068-023-01380-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-023-01380-4