Abstract
Rosa davurica is widely used to treat various kinds of diseases because of its high antioxidant, antiviral and anti-inflammatory activities. This use of plant-based materials as medicine is called phytomedicine and has been widely practiced since time immemorial. However, the pharmacological mechanism of R. davurica in skin photoaging is not yet fully understood. Therefore, this study was carried out to evaluate the recovery effects of R. davurica leaf extracts (RDE) in UVB-irradiated human skin keratinocytes (HaCaTs) and investigate whether RDE is a potential therapeutic agent against skin photoaging. The expression of aging-related markers including mitogen-activated protein kinases/activator protein 1 (MAPK/AP-1), nuclear factor-κB (NF-κB), and nuclear factor E2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) was evaluated using Western blot analysis. The reactive oxygen species (ROS) was also used by FACS in HaCaTs. Findings indicated that RDE is efficient in scavenging free radicals and dose-dependently reducing ROS generation. Furthermore, RDE notably decreased UVB-induced matrix metalloproteinase-1 (MMP-1) expression through inhibition of MAPK/AP-1 and NF-κB signaling pathways as well as induced blocking of extracellular matrix (ECM) degradation in UVB-irradiated HaCaTs. In addition, RDE improved Nrf2/HO-1 signaling that increases oxidative defense capacity and enhances transforming growth factor-beta (TGF-β) signaling activation to promote procollagen type I synthesis, relieving UVB-induced skin cell damage. In conclusion, the protective effects of RDE on skin cellular components suggest that it has a high biological potential for skin protection from UVB-induced skin photoaging and is a good candidate for drug and cosmetic application.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Skin aging is characterized by loss of structure, wrinkles, pigmentation, drying, and degradation of collagen, and physiological functions [1]. It occurs due to two biochemical processes, intrinsic and extrinsic (photoaging) aging. Intrinsic aging is an inevitable process that occurs over time and is highly correlated with genetic factors. In contrast, extrinsic aging is caused by several hazardous environmental factors [2]. Therefore, the skin serves as the first line of defense protecting the body against extrinsic factors and is continuously in contact with toxic chemicals, infectious agents, and ultraviolet radiation [3].
UVB exposure evokes complex and multifaceted signal networks that promote the intracellular formation of ROS, which in turn damages lipids, proteins, and nucleic acids in keratinocytes. In addition, UV exposure increases epidermal thickness, reduces collagen synthesis, and facilitates collagenolysis by upregulating the expression of matrix metalloproteases (MMPs) [4]. UVB-induced ROS activates mitogen-activated protein kinase (MAPK) signaling that further induces MMPs and eventually promotes collagen degradation [5, 6].
In the skin, impairment of type I procollagen is dependent on the expression of MMP-1, which is a zinc-dependent endopeptidase that degrades type I collagen specifically. It is mainly regulated by the upper signaling cascades, such as MAPKs, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 [7]. After activation of the MAPKs by UV, transcription factor (AP-1) expression increases, leading to collagen fragmentation, inflammatory response, and cell death [8, 9]. In addition, NF-κB, another critical transcription factor of MMP in the dermis, is translocated under photodamaged conditions into the nucleus by degradation of IκB and elevates MMP and pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [10, 11]. Interestingly, UVB alters not only the collagen degradation pathway but also the synthesis pathway, TGFβ/Smad. The TGFβ/Smad pathway involves reduction of TβRII level, and phosphorylation of Smad3 the downstream signaling is significantly decreased in photoaged human skin [12, 13]. Impairment of the TGFβ/Smad pathway is also one of the mechanisms causing the loss of collagen. Due to complicated changes in various pathways, the ECM collapses, resulting in wrinkle formation.
Several studies on skin damage by UVB have been done focusing on anti-photoaging. Currently, research is focusing on identifying natural products and organic compounds. with anti-photoaging effects. Rosa davurica is a herbal plant occuring in Korea, China, and Japan [14]. It has long been used as a traditional Chinese herbal therapy for diseases because of its antioxidant activities, anti-HIV, anti-viral, anti-biotic, and hypoglycemic activities [15, 16]. In addition, R. davurica improves DNCB-induced atopic dermatitis [17]. Although R. davurica is known for various efficacies, it’s anti-photoaging effect is not yet well understood. Therefore, we investigate R. davurica’s anti-photoaging effects and its molecular mechanism through regulating MAPK/AP-1, NF-κB, and Nrf2/HO-1 Signaling on UVB-irradiated human keratinocytes (HaCaTs).
2 Materials and methods
2.1 Chemicals and reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin were supplied by Gibco RBL (Grand Island, NY, USA). Standard apigenin, positive control ascorbic acid, dexamethasone, and 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Human Total MMP-1 and Human Total MMP-3 ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA). Organic solvents were purchased from Samchun Chemical (Seoul, Korea) and Daejung Chemical & Metal (Siheung, Korea). Inorganic salts were purchased from Sigma-Aldrich. Silica gel was purchased from Merck (Kenilworth, NJ, USA). The primary and secondary antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA), Santa Cruz Biotechnology (Santa Cruz, CA, USA), and Bio-Rad Laboratories, Inc. (Hercules, CA, USA).
2.2 Sample preparations
The leaf of R. davurica was provided from Wonsamrosehip Co., Ltd. (Gyeonggi-do, Korea) and was confirmed genetically by DNA analysis. The voucher specimen is kept in the Snowwhitefactory Co, Ltd. laboratory. The dried leaf of R. davurica was etracted using 30% (v/v) aqueous ethanol for 24 h at room temperature. After being filtered through 5 μm filters, the extract was concentrated and lyophilized. The yield of extract was 20.9%.
2.3 Total phenolic content and total flavonoid content
The total phenolic content (TPC) of RDE, was quantified based on Folin–Ciocalteau colorimetric method [18]. Also, the total flavonoid content (TFC) of RDE was evaluated based on the aluminum chloride (AlCl3) colorimetric method [19].
2.4 HPLC anaysis
The phytochemical characteristics of RDE and the standard compounds ethyl gallate and ellagic acid were identified by high performance liquid chromatography (HPLC). The HPLC analysis was performed on Waters e2695 system with 2998 PDA detector (Millford, MA, USA) with an Atlantis T3 column (4.6 mm X 250 mm, 5 μm, Waters, Millford, MA, USA). The mobile phase consisted of (A) water and (B) acetonitrile both containing 0.1% formic acid with gradient elution: 8% B (0–10 min), 8–30% B (10–35 min), 30% B (35–40 min), 30–60% B (40–45 min), 60% B (45–50 min), 60–100% (50–60 min); then keeping 100% B for 5 min to clean the column, and the re-equilibrating step of the column was 8% B isocratic for 5 min. The flow rate was 1.0 mL/min, and the injection volume was 10 μl. The samples were analyzed at a wavelength of 280 nm and the column temperature was maintained at 40 ℃.
2.5 DPPH radical scavenging activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH, PubChem CID: 2,375,032) assay was used for scavenging activity against free radical. The absorbance of 0.1 mM DPPH solution in 80% (v/v) methanol was set at 0.650 ± 0.02 at 517 nm. The reaction of the mixture of DPPH solution and diluted sample with different concentrations (satisfied the standard curve) were processed at 37 ℃ for 30 min. The descent of absorbance resulting of radical scavenging capacity and were read using microplate reader at 595 nm. The DPPH radical scavenging activity of the sample was evaluated using the following formula:
whereas: ODo negative control, ODx various tested RDE and ascorbic acid concentrations.
2.6 ABTS radical scavenging activity
The 2,2-azino-bis-(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS, PubChem CID: 5,464,076) assay was determined using reagent. The protocol was followed from the previous study with a slight modification. The reaction of the mixture of ABTS solution and diluted sample with different concentrations (satisfied the standard curve) were processed at 37 ℃ for 10 min. The reduction of blue green ABTS+ resulting of hydrogen-donating antioxidant and were read using microplate reader at 405 nm. The ABTS radical scavenging activity of the sample was evaluated using the following formula:
whereas: ODo negative control, ODx various tested RDE and ascorbic acid concentrations.
2.7 Cell culture
HaCaT cells, originating from human keratinocytes, were obtained from Sciencell (Carlsbad, CA, USA). Cells were cultured with DMEM medium supplemented with 10% heat-inactivated FBS and 1% penicillin–streptomycin (Gibco BRL, Grand Island, NY, USA) at 37 ℃ in the atmosphere containing 5% CO2. When the population reached 90–95% confluency, the cells were seeded in 6/96-well plates and 35 mm/100 mm dishes.
2.8 Cell viability
The effects of RDE on the viabilities of HaCaT cells were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St-Louis, MO, USA) assay. MTT is a colorimetric assay for evaluating cell viability. The cell survival was initiated through measuring the absorbance of the colored solution at a certain wavelength. After 48 h treatment supernatant of each well was removed entirely and a total of 100 µL MTT solution (0.1 mg/mL) was added to each well. After 4 h of incubation, the supernatants were aspirated and 150 µL dimethyl sulfoxides were added to each well. The absorbance at 570 nm was measured by a microplate reader (Molecular Devices FilterMax F5; San Francisco, CA, USA).
2.9 UVB irradiation and samples treatment
For UVB irradiation, the cells were rinsed twice with phosphate buffered saline (PBS) and then were exposed to UVB light with a dose of 125 mJ/cm2 generated from a UVB radiation machine (Bio-Link BLX-312; Vilber Lourmat GmbH, France). After UVB irradiation, the cells were washed twice with PBS and immediately treated with or without samples in serum-free medium conditions. The normal/control group was kept in the same culture conditions without samples and undergo UVB irradiation. 10 µM ascorbic acid (positive control) or three doses of RDE, fraction and isolation component were added to each plate for incubation.
2.10 Measurement of ROS production
The 2,7-dichlorofluorescein diacetate (DCFH-DA) was used to detect intracellular ROS levels. After 24 h samples treatment, the cells were stained with 30 µM 2,7-dichlorofluorescein diacetate (DCF-DA) (Sigma-Aldrich) for 30 min at 37 ℃ in a CO2 incubator. The cells were collected and then analyzed by flow cytometry (FACSCalibur™; Becton–Dickinson, San Jose, CA, USA).
2.11 Enzyme-linked immunosorbent assay
After 24 h samples treatment, the supernatants were harvested. The secretion of MMP-1, MMP-3 and IL-6 were detected with commercially available ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA) following the manufacturer’s instruction.
2.12 Reverse transcriptase (RT)-PCR
After 24 h of incubation treatments total RNA was isolated from HaCaT cells according to the manufacturer’s instructions by TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA). The RNA (3 µg) was reverse transcribed with 200 units of reverse transcriptase and 0.5 µg/µL oligo-(dT)15 primer (Bioneer Co., Daejeon, Korea). This reaction was performed at 70 ℃ for 5 min, 42 ℃ for 60 min, and was terminated at 94 ℃ for 5 min. Amplified products were observed by gel electrophoresis and detected by nucleic acid staining (NobleBio Inc., Korea) under UV illumination. GAPDH was used for normalization.
2.13 Western blot analysis
For western blot analysis, the total proteins were lysed using ice-cold lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1.0% Triton X-100, 1.0% sodiumdexycholate, 0.1% SDS and so on) for 1 h and centrifuged at 12,000 g for 20 min. After centrifugation, the proteins were quantified with Bradford reagent (Bio-Rad, Hercules, CA, USA). 50 μg of proteins were separated on 10–15% sodium dodecyl sulfate–polyacrylamide gels (SDS-PAGE) and then transferred onto a nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK). Subsequently, the membrane that contained the transferred proteins was blocked in 5% nonfat dry milk in TBST (50 mM Tris–HCL, 150 mM NaCl and 0.1% Tween 20, PH 7.4) for 1 h at room temperature and then incubated with appropriate primary antibody in TBST overnight at 4 ℃. After incubation with the primary antibody, the membrane was washed three times by TBST buffer and then incubated with the corresponding secondary antibody for 1 h at room temperature. Protein bands were detected using chemiluminescence detection electrochemiluminescence (ECL) reagents (Fujifilm, LAS-4000, Tokyo, Japan). Densitometric measurements of bands were analyzed by ImageMasterTM 17 2D Elite software version 3.1 (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
2.14 Statistical analysis
The data were analyzed using Statistical Analysis System (GraphPad Prism 5). All the quantitative data are represented as the means ± SD. The significant differences were determined using a one-way analysis of variance (ANOVA) with the Student–Newman–Keuls test for the multiple comparisons. p < 0.05, p < 0.01 and p < 0.001 were considered statistically significant for t test. All experiments were performed independently three times.
3 Results
3.1 Analysis of chemical contents of RDE
RDE had high contents of total phenols, and flavonoid of 176.0 ± 0.6 mg gallic acid/g extract, 173.7 ± 1.2 mg catechin/g extract, respectively. And the HPLC analysis was performed as described in the Materials and Methods. Ethyl gallate and ellagic acid were used as authentic standards, and their contents in the RDE were determined to be 25.20 ± 0.19 and 8.79 ± 0.2 (mg/g extract), respectively Fig. 1.
3.2 Antioxidative activities of RDE
To evaluate the antioxidant potential of RDE, DPPH, and ABTS assays were performed. Ascorbic acid was used as a positive control because it has free radical scavenging activity. Findings revealed that RDE at different concentrations (1, 10, 50, 100 and 250 µg/mL) has free radical scavenging activities on DPPH (IC50 of 22.8 µg/mL) and ABTS (IC50 of 19.2 µg/mL), which congruently increases in a dose-dependent manner. The presence of RDE free radical scavenging activity is consistent with its high antioxidant activity. The antioxidant acts as a preventive agent or delays the mechanism of some types of cell damage.
3.3 Effect of RDE on cell viability
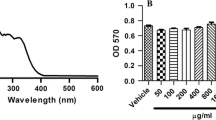
Results of cytoprotective effects of UVB-irradiated cells treated with RDE (0.1–50 µg/mL) indicated that UVB irradiation at 125 mJ/cm2 has slight cytotoxicity (Fig. 2). Furthermore, cells treated with RDE had lower cytotoxicity than UVB radiation group. Based on these results, a range of RDE of 1–50 µg/mL was used for the subsequent experiments.
3.4 Effect of RDE on intracellular ROS production by FACS
ROS generation plays an essential role in UVB-irradiated cellular signaling [20]. The intracellular ROS production was measured using flow cytometry and ROS scavenging activities of RDE were assessed by the fluorescence dye, DCFH-DA assay. DCFH-DA is hydrolyzed by intracellular esterase and is changed to nonfluorescent DCFH, which is oxidized to highly fluorescent DCF in the presence of ROS. The fluorescent signal increased after UVB radiation (84.1% of UVB-irradiated group). However, treatment with RDE significantly reduced UVB-induced upregulation of fluorescent intensity to 41.7%.
3.5 Effect of RDE on MMP-1, MMP-3 and IL-6 production measured by ELISA
The secretion of MMP-1, MMP-3, and IL-6 was measured to confirm the effects of RDE on cells anti-aging activity. Cells were exposed to UVB 125 mJ/cm2 radiation prior to treatment with indicated concentrations of RDE at 1, 10, and 50 μg/mL. After 24 h, supernatants were collected and measured using ELISA kits. UVB irradiation significantly increased MMP-1, MMP-3, and IL-6 secretion by 33.6, 32.9 and 1005.9%, respectively (Fig. 3). However, the presence of RDE significantly reversed these trends. Compared with UVB-irradiated cells, RDE inhibited MMP-1 secretion by 31.7% at 50 μg/mL. Also, RDE downregulated the levels of MMP-3 and IL-6 in a dose-dependent manner, leading to a decrease in release of MMP-3 and IL-6 by 46.5 and 44.7% at 50 μg/mL, respectively.
3.6 Effect of RDE on mRNA expression of MMP-1 and Type I procollagen analyzed by RT-PCR
An increase in MMPs and a decrease in procollagen are the primary causes of skin photoaging [21]. To make the photoprotective effects of RDE clear, the mRNA levels of MMP-1 and procollagen type Iwere further measured by RT-PCR. In agreement with ELISA test results, the mRNA level of MMP-1 in UVB-irradiated HaCaTs was also markedly elevated, compared with that of non-irradiated cells, whereas RDE decreased UVB-induced MMP-1 by 66.5%, compared with the UVB-irradiated group (Fig. 4A). In contrast, UVB irradiation inhibited type I procollagen mRNA expression, compared with that of the normal group. Interestingly, RDE reversed UVB-induced type I procollagen reduction in a dose-dependent manner (Fig. 4B). Based on the above findings, RDE showed the best effect on UVB-induced photodamage. Therefore, RDE were used to further explored their biological potential.
Intracellular ROS on UVB‐irradiated HaCaT cells from RDE. The intracellular ROS levels were quantitatively determined via flow cytometry (A). Following UVB irradiation (125 mJ/cm2), HaCaTs were treated with or without the indicated concentration of RDE (1, 10, and 50 μg/mL) for 24 h. ROS levels were measured by DCFH-DA assay. The relative intensity of DCF fluorescence is shown in the bar graph. The results were shown as the mean ± SD of three independent experiments. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with non-irradiated group, *p < 0.05, **p < 0.01, ***p < 0.001 compared with only UVB-irradiated group
3.7 Effect of RDE on UV-B induced AP-1 signaling pathway
AP-1 as a transcription factor could regulate MMPs gene expression in response to UVB radiation [22]. To explore the inhibitory mechanism of RDE on MMP-1 expression, the expression of the major components of AP-1 (c-fos and c-jun, as well as their phosphorylation) were quantified. For the cells subjected to UVB radiation, the expression of p–c-fos and p–c-jun were significantly higher than that of the normal group. The elevated p–c-fos and p–c-jun expression caused by UVB were significantly decreased using RDE treatment. RDE at 10 and 50 µg/mL suppressed UVB-induced p–c-fos by 10.4 and 49.8%, and p–c-jun by 23.7 and 56.4%, respectively (Fig. 5) as compared to the UVB-irradiated group. These significant findings suggested that RDE inhibited MMP-1 expression through regulation of AP-1 transcriptional factor.
MMP-1, -3 and IL-6 secretion on UVB-irradiated HaCaT cells from RDE. Following UVB irradiation (125 mJ/cm2), HaCaTs were treated with or without the indicated concentration of RDE (1, 10, and 50 μg/mL) for 24 h. MMP-1 secretion (A), MMP-3 secretion (B), IL-6 secretion (C) in UVB-irradiated HaCaTs were measured by ELISA kits. The results were shown as the mean ± SD of three independent experiments. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with non-irradiated group, *p < 0.05, **p < 0.01, ***p < 0.001 compared with only UVB-irradiated group
3.8 Effect of RDE on UV-B induced MAPK signaling pathway
The MAPK is a ROS-sensitive signal pathway, which plays a key role in activation of AP-1 transcription factor and MMPs expression [23]. The expression of the phosphorylated forms of the MAPKs family, including p-JNK, p-p38, and p-ERK were explored to investigate the effects of RDE on UVB-induced photoaging. As shown in Fig. 6, UVB irradiation significantly increased the expression of p-JNK, p-p38, and p-ERK in HaCaTs, whereas RDE significantly reduced the activation of the MAPK signaling pathway. Compared with UVB-induced HaCaTs, RDE dose-dependently decreased the phosphorylation of ERK, JNK, and p38. Furthermore, at 50 µg/mL, RDE were able to reduce the expression of p-ERK, p-JNK and p-p38 by 37.7, 53.7 and 61.0%, respectively Fig. 7.
MMP-1 and Procollagen type I mRNA expression on UVB-irradiated HaCaT cells from RDE. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with RDE (1, 10 and 50 μg/mL) for 24 h. MMP-1 and procollagen type I mRNA levels were determined by RT-PCR (A). The band intensities were quantified by densitometry and normalized to the non-irradiated group (B). The results were shown as the mean ± SD of three independent experiments. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with non-irradiated group, *p < 0.05, **p < 0.01, ***p < 0.001 compared with only UVB-irradiated group
AP-1 signaling pathway on UVB-irradiated HaCaT cells from RDE. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with RDE (10 and 50 μg/mL) for 4 h. Phosphorylated and non-phosphorylated forms of c-Fos and c-Jun were detected by Western blot (A). The band intensities were quantified by densitometry, normalized to the level of c-Jun (B) and c-fos (C), and calculated as the percentage of the non-irradiated group. The results were shown as the mean ± SD of three independent experiments. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with non-irradiated group, *p < 0.05, **p < 0.01, ***p < 0.001 compared with only UVB-irradiated group
3.9 Effect of RDE on UV-B induced NF-κB signaling pathway
The NF-κB pathway is another key intermediary in UVB-induced MMPs expression [24]. Effects of RDE on p-NF-κB in UVB-irradiated HaCaTs were explored. Results indicated that RDE effectively inhibited UVB-induced phosphorylation of NF-κB. As shown in Fig. 5, RDE (10 µg/mL) decreased the p-NF-κB expression by 53.8%.
3.10 Effect of RDE on UV-B induced TGF-β signaling pathway
To determine if RDE promoted type I procollagen synthesis by activation of TGF-β signaling pathway, the effects of RDE on the levels of TGF-β1, procollagen type I and MMP-1 were further investigated in UVB-irradiated HaCaTs. Results show that UVB irradiation significantly decreased TGF-β1 and procollagen type I but increased MMP-1 expression. The RDE dose-dependently enhanced UVB-induced TGF-β1 and procollagen type I, attenuated MMP-1 through downregulation. As shown in Fig. 10, 50 µg/mL RDE significantly increased the expression of TGF-β1 and procollagen type I proteins by 16.7 and 55.3%, respectively. On the other hand, it decreased the expression of MMP-1 proteins by 50.4%, compared with the UVB group. These results suggest that RDE protected procollagen reduction by activation of the TGF-β signaling pathway.
3.11 Effect of RDE on UV-B induced Nrf2/ARE signaling pathway
Another possible mechanism of anti-photoaging effects of RDE can be the regulation of the endogenous antioxidant system. Nrf2 is an important cellular defense system that induces numerous antioxidant-related gene expression such as HO-1 and NQO-1 [25]. Here, the effects of RDE on the protein levels of Nrf2, HO-1 and NQO-1 were evaluated in UVB-irradiated HaCaTs. The results indicate that exposure of cells to UVB radiation significantly increased the expression of Nrf2 and promoted the levels of HO-1 and NQO-1, which could be attributed to physiological self-protection. UVB-induced Nrf2 was further elevated by RDE treatment. As shown in Fig. 11, 50 µg/mL RDE could increase Nrf2 expression by 53.8% in UVB-irradiated HaCaTs, compared with the UVB group. The levels of HO-1 and NQO-1 were also enhanced when cells were treated with RDE. A concentration of 50 µg/mL RDE significantly promoted the expression of HO-1 and NQO-1 by 111.7 and 136.7%, respectively.
MAPK signaling pathway on UVB-irradiated HaCaT cells from RDE. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with RDE (10 and 50 μg/mL) for 1 h. Phosphorylated and non-phosphorylated forms of ERK, JNK and P38 were detected by Western blot (A). The band intensities were quantified by densitometry, normalized to the level of ERK, JNK and P38, and calculated as the percentage of the non-irradiated group (B). The results were shown as the mean ± SD of three independent experiments. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with non-irradiated group, *p < 0.05, **p < 0.01, ***p < 0.001 compared with only UVB-irradiated group
4 Discussion
Phytochemicals have been used by mankind in a wide variety of ways since time immemorial. As stated by Yadav and Agarwala, the World Health Organization claimed that medicinal plants are an ideal source of drugs [26]. Around 80% of individuals in developed countries use traditional medicines based on compounds derived from medicinal plants. One of the widely used plants for medicinal purposes is the R. davurica. The genus R. davurica, with about 100 species, occurs in the north of China, Japan, and Korea, many of which exhibit numerous pharmacological properties [15, 27]. As a traditional or folk medicine, R. davurica has long been used to treat various diseases, such as dyspepsia, gastroenterology, arteriosclerosis, pulmonary tuberculosis, frostbite, and menoxenia. Modern pharmacological research has revealed that R. davurica exhibits antioxidant, anti-inflammatory, anti-angiogenic, anti-allergy, anti-nociceptive activities, anti-lipid peroxidation effect, and inhibitory activity against neutrophil migration [28]. However, there has been no study on the skin anti-photoaging effects of the R. davurica. Thus, this study was done determine if RDE inhibited UVB‐induced photoaging via inhibition of the MAPK/AP‐1, NF-κB, and Nrf2/HO-1 pathway.
Oxidative stress caused by the factors considered in this study leads to increased ROS generation and reduced antioxidant capacity, resulting in aging and visible deterioration in skin condition [29, 30]. Our results indicate that the inhibitory activity of DPPH and ABTS free scavenging radicals was detectable at IC50 values of 22.8 µg/mL and 19.2 µg/mL, respectively (Fig. 9). After treatment with RDE, the level of ROS significantly dose-dependently decreased (Fig. 10). The ability of RDE to scavenge DPPH radicals was similar to that of ascorbic acid, a well-known skin brightener that was used as a positive control.
NF-κB signaling pathway on UVB-irradiated HaCaT cells from RDE. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with RDE (10 and 50 μg/mL) for 4 h. NF-κB protein expression were detected by Western blot (A). The band intensities were quantified by densitometry, normalized to the level of β-actin, and calculated as the percentage of the non-irradiated group (B). The results were shown as the mean ± SD of three independent experiments. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with non-irradiated group, *p < 0.05, **p < 0.01, ***p < 0.001 compared with only UVB-irradiated group
TGF-β1 signaling pathway on UVB-irradiated HaCaT cells from RDE. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with RDE (10 and 50 μg/mL) for 1.5 h. TGF-β1, procollagen type I and MMP-1 protein expression was detected by Western blot (A). The band intensities were quantified by densitometry, normalized to the level of β-actin, and calculated as the percentage of the non-irradiated group (B, C). The results were shown as the mean ± SD of three independent experiments. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with non-irradiated group, *p < 0.05, **p < 0.01, ***p < 0.001 compared with only UVB-irradiated group
Also, UV radiation-induced ROS triggers an increase in MMPs, degrades ECM proteins such as collagen and elastin, and leads to skin photoaging and wrinkle formation [31]. To evaluate the effect of pre-and post-enzyme-treated RDE on UVB-induced photoaging, we investigated the mRNA expression of MMP-1 and procollagen type I in UVB-irradiated HaCaT cells using RT-PCR. Our results demonstrated that RDE recovered the expression of procollagen type I (Fig. 4) and significantly decreased MMP-1 expression in UVB-irradiated HaCaTs. These findings suggest that RDE prevents collagen loss through the inhibition of UVB-induced MMP-1 expression and the enhancement of procollagen type I expression.
The main factors that defend skin cells from oxidative stress are Nrf2 and several of its target genes, such as NQO1 and HO-1 [32]. Kannan and Jaiswal [33] found that Nrf2 upregulated ARE-mediated gene expression in UVB exposed fibroblasts. Our results also show that activation of both Nrf2 and its target genes, such as NQO1 and HO-1, is due to UVB-triggered ROS signaling. In skin subjected to extrinsic aging by UVB irradiation, RDE led to decreased oxidative stress by regulating the Nrf2/ARE signaling pathway via the expression of antioxidant enzymes such as NQO1 and HO-1 (Fig. 8).
Keratinocytes, the predominant cell type in the epidermis, absorb the majority of UVB irradiation, leading to dryness, loosing, and inflammation [34]. The UVB irradiation of the epidermis results in ROS generation, which increased MMP-1, MMP-3, and IL-6 secretion. In this study, RDE treatment in HaCaTs while undergoing UVB irradiation resulted in decreases in MMP-1, MMP-3, and IL-6 secretion. Hwang et al. [17] reported low cytokine levels in groups treated for atopic dermatitis with leaf extracts of R. davurica. IL-6 plays a key role in the inflammatory responses of HaCaTs following UVB exposure, and as shown in our data, treatment with RDE can regulate IL-6 production. After UVB irradiation, RDE-treated cells showed TGF-β1 and type I procollagen (Fig. 3, 7).
The MAPK signaling pathway plays a key role in regulating MMPs expression, cell proliferation, differentiation, and death [35]. The major function of MAPK is transferring extracellular signals to the nucleus, thereby activating transcription factors and inducing target gene expression. AP-1, as a MAPK downstream activator, is a well-known transcription factor that stimulates gene transcription of MMPs [36]. To explore the underlying mechanism of RDE, we performed further studies on the MAPK/AP-1 signaling pathway in HaCaTs (Fig. 5, 6). RDE showed that the deglycosylated products exhibited stronger activity than their respective counterparts in inhibiting UVB radiation-induced phosphorylation of ERK, JNK, p-38, as well as p–c-fos, and p–c-jun expression in HaCaT cells. Lu Li et al. [37] and Choi HJ et al. [38] reported UVB-induced MAPK/AP-1 expression in HaCaTs, similarly our results indicate that RDE regulates MAPK and AP-1 signaling pathways.
Aside from reducing collagen production in the skin, UVB-irradiated keratinocytes also overexpressed certain inflammatory cytokines [39]. NF-κB is responsible for activating the transcription of inflammatory cytokines. When combined with its inhibitory protein IκB-α, NF-κB commonly exists in an inactivated state in the cytoplasm. Once stimulated, degraded IκB-α leads to the nuclear localization of NF-κB, inducing the expression of a variety of inflammatory factors like IL-6 [40]. Moreover, inflammatory cytokine-mediated inflammation increases ROS generation and further amplifies UV-induced skin damage [41]. Thus, the NF-κB pathway plays a central role in skin aging and the inflammatory generation. We further measured the expression of NF-κB and IκB-α by Western blot to verify the mechanisms of RDE. Hwang E et al. [42] and Zhang M et al. [43] reported that UVB-induced damage is controlled through the regulation of the NF-κB pathways. Similar to the findings of the above studies, we found that the RDE inhibited the UVB-induced promoter activity of NF-κB and degradation of IκB-α to varying degrees (Fig. 11).
Nrf2/ARE signaling pathway on UVB-irradiated HaCaT cells from RDE. HaCaTs were irradiated with UVB (125 mJ/cm2), followed by treatment with RDE (10 and 50 μg/mL) for 3 h. Total HO-1 and NQO-1 (A) and nucleus Nrf2 (B) expression were detected by Western blot. The band intensities of total HO-1 and NQO-1 (C), nucleus Nrf2 (D) were quantified by densitometry, normalized to the level of β-actin and histone, and calculated as the percentage of the non-irradiated group. The results were shown as the mean ± SD of three independent experiments. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with non-irradiated group, *p < 0.05, **p < 0.01, ***p < 0.001 compared with only UVB-irradiated group
Overall, we found that RDE exhibited a superior ability to inhibit MMPs and IL-6 production by regulating the MAPK/AP-1, NF-κB/IκB-α pathways in UVB-irradiated HaCaTs. Moreover, RDE increases cytoprotective antioxidants such as HO-1 and NQ-O1 expression by facilitating the nuclear accumulation of Nrf2. Therefore, we conclude that the RDE have great potential for use against skin photoaging.
5 Conclusions
In summary, we demonstrated that RDE effectively protected HaCaTs against UVB‐induced photodamage by decreasing ROS generation, MMPs, and IL-6. The relative protection mechanism involved inhibition of the MAPK/AP‐1 and NF-κB pathways. Moreover, RDE extract improved Nrf2/HO-1 signaling that procollagen type I synthesis by increasing the level of TGF‐β1. All these results suggest that RDE on skin cellular components suggest that it has a high biological potential for skin protection from UVB-induced skin photoaging and is a good candidate for drug and cosmetic application. However, evaluation of the biological effect of RDE and its active compounds on in vivo models and clinical trials should be further clarified.
Supplementary Materials: Table S1: Oligonucleotide primers used for RT-PCR.
Data availability statement
The data presented in this study are available in this paper.
Sample availability
Samples are not available.
References
Zhang, S., & Duan, E. (2018). Fighting against skin aging: the way from bench to bedside. Cell Trans, 27(5), 729–738. https://doi.org/10.1177/0963689717725755
Shin, J. W., Kwon, S. H., Choi, J. Y., Na, J. I., Huh, C. H., Choi, H. R., & Park, K. C. (2019). Molecular mechanisms of dermal aging and antiaging approaches. Intern J Mole Sci, 20(9), 2126. https://doi.org/10.3390/ijms20092126
Quaresma, J. A. S. (2019). Organization of the skin immune system and compartmentalized immune responses in infectious diseases. Clinical Microbiology Reviews, 32(4), e00034-e118. https://doi.org/10.1128/CMR.00034-18
de Jager, T. L., Cockrell, A. E., & Du Plessis, S. S. (2017). Ultraviolet light induced generation of reactive oxygen species. Advances in Experimental Medicine and Biology, 996, 15–23. https://doi.org/10.1007/978-3-319-56017-5_2
Kwon, K. R., Alam, M. B., Park, J. H., Kim, T. H., & Lee, S. H. (2019). Attenuation of UVB-induced photo-aging by polyphenolic-rich spatholobus suberectus stem extract via modulation of MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients, 11(6), 1341. https://doi.org/10.3390/nu11061341
Tanaka, Y., Uchi, H., & Furue, M. (2019). Antioxidant cinnamaldehyde attenuates UVB-induced photoaging. Journal of Dermatological Science, 96(3), 151–158. https://doi.org/10.1016/j.jdermsci
Jin, Y. J., Ji, Y., Jang, Y. P., & Choung, S. Y. (2021). Acer tataricum subsp ginnala inhibits skin photoaging via regulating MAPK/AP-1, NF-κB, and TGFβ/smad signaling in UVB-irradiated human dermal fibroblasts. Molecules, 26(3), 662. https://doi.org/10.3390/molecules26030662
Munshi, A., & Ramesh, R. (2013). Mitogen-activated protein kinases and their role in radiation response. Genes & Cancer, 4, 401–408. https://doi.org/10.1177/1947601913485414
Karin, M. (1995). The regulation of AP-1 activity by mitogen-activated protein kinases. Journal of Biological Chemistry, 270, 16483–16486. https://doi.org/10.1074/jbc.270.28.16483
Cooper, S., & Bowden, G. (2007). Ultraviolet B regulation of transcription factor families: roles of nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Current Cancer Drug Targets, 7, 325–334. https://doi.org/10.2174/156800907780809714
Li, N., & Karin, M. (1998). Ionizing radiation and short wavelength UV activate NF-κB through two distinct mechanisms. Proc Natl Acad Sci Unit State Am, 95, 13012–13017. https://doi.org/10.1073/pnas.95.22.13012
He, T., Quan, T., Shao, Y., Voorhees, J. J., & Fisher, G. J. (2014). Oxidative exposure impairs TGF-β pathway via reduction of type II receptor and SMAD3 in human skin fibroblasts. Age, 36, 9623. https://doi.org/10.1007/s11357-014-9623-6
Quan, T., He, T., Voorhees, J. J., & Fisher, G. J. (2001). Ultraviolet irradiation blocks cellular responses to transforming growth factor-β by down-regulating its type-II receptor and inducing Smad7. Journal of Biological Chemistry, 276, 26349–26356. https://doi.org/10.1074/jbc.M010835200
Jung, H. J., Sa, J. H., Song, Y. S., Shim, T. H., Park, E. H., & Lim, C. J. (2011). Anti-inflammatory, anti-angiogenic, and anti-nociceptive activities of the chloroform fraction of a methanol extract from Rosa davurica Pall. Leaves in experimental animal models. Immunopharmacology and Immunotoxicology, 33(1), 186–192. https://doi.org/10.3109/08923973.2010.491516
Tong, H., Jiang, G., Guan, X., Wu, H., Song, K., Cheng, K., & Sun, X. (2016). Characterization of a polysaccharide from Rosa davurica and inhibitory activity against neutrophil migration. Intern J Biol Macromole, 89, 111–117. https://doi.org/10.1016/j.ijbiomac
Kim, H. M., Park, Y. A., Lee, E. J., & Shin, T. Y. (1999). Inhibition of immediate-type allergic reaction by Rosa davurica Pall. In a murine model. Journal of Ethnopharmacology, 67(1), 53–60. https://doi.org/10.1016/s0378-8741(99)00013-6
Hwang, D. H., Koh, P. O., Kang, C., & Kim, E. (2021). Rosa davurica Pall. Improves DNCB-induced atopic dermatitis in mice and regulated TNF-Alpa/IFN-gamma-induced skin inflammatory responses in HaCaT cells. Phytomedicine, 91, 153708. https://doi.org/10.1016/j.phymed
Yoshida, T., Zhe, X. J., & Okuda, T. (1989). Taxifolin apioside and davuriciin M1, a hydrolysable tannin from Rosa davurica. Phytochemistry. https://doi.org/10.1016/s0031-9422(00)97939-1
Wilson, I. D., Plumb, R., Granger, J., Major, H., Williams, R., & Lenz, E. M. (2005). HPLC-MS-based methods for the study of metabonomics. J Chromatogr Anal Technol Biomed Life Sci, 817(1), 67–76. https://doi.org/10.1016/j.jchromb
Kulms, D., Zeise, E., Pöppelmann, B., & Schwarz, T. (2002). DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene, 21(38), 5844–5851. https://doi.org/10.1038/sj.onc.1205743
Pittayapruek, P., Meephansan, J., Prapapan, O., Komine, M., & Ohtsuki, M. (2016). Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Intern J Mole Sci, 17(6), 868. https://doi.org/10.3390/ijms17060868
Lu, J., Guo, J. H., Tu, X. L., Zhang, C., Zhao, M., Zhang, Q. W., & Gao, F. H. (2016). Tiron inhibits UVB-induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PLoS ONE, 11(8), e0159998. https://doi.org/10.1371/journal.pone.0159998
Guo, Y., Pan, W., Liu, S., Shen, Z., Xu, Y., & Hu, L. (2020). ERK/MAPK signalling pathway and tumorigenesis (Review). Exper Therapeutic Med, 19(3), 1997–2007. https://doi.org/10.3892/etm.2020.8454
Tanaka, K., Hasegawa, J., Asamitsu, K., & Okamoto, T. (2005). Prevention of the ultraviolet B-mediated skin photoaging by a nuclear factor kappaB inhibitor, parthenolide. J Pharm Exper Ther, 315(2), 624–630. https://doi.org/10.1124/jpet.105.088674
Ma, Q. (2013). Role of nrf2 in oxidative stress and toxicity. Ann Rev Pharm Toxicol, 53, 401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Yadav, R. N. S., & Agarwala, M. (2011). Phytochemical analysis of some medicinal plants. J Phytol., 3(12), 10–14.
Cavinato, M., Waltenberger, B., Baraldo, G., Grade, C. V. C., Stuppner, H., & Jansen-Dürr, P. (2017). Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontol, 18(4), 499–516. https://doi.org/10.1007/s10522-017-9715-7
Huo, Y., Gao, Y., Mi, J., Wang, X., Jiang, H., & Zhang, H. (2017). Isolation and Simultaneous Quantification of Nine Triterpenoids from Rosa davurica Pall. Journal of Chromatographic Science, 55(2), 130–136. https://doi.org/10.1093/chromsci/bmw155
Rinnerthaler, M., Bischof, J., Streubel, M. K., Trost, A., & Richter, K. (2015). Oxidative stress in aging human skin. Biomolecules, 5(2), 545–589. https://doi.org/10.3390/biom5020545
Liu, Z., Ren, Z., Zhang, J., Chuang, C. C., Kandaswamy, E., Zhou, T., & Zuo, L. (2018). Role of ROS and nutritional antioxidants in human diseases. Frontiers in Physiology, 9, 477. https://doi.org/10.3389/fphys.2018.00477
Gromkowska-Kępka, K. J., Puścion-Jakubik, A., Markiewicz-Żukowska, R., & Socha, K. (2021). The impact of ultraviolet radiation on skin photoaging—review of in vitro studies. J Cosmetic Dermatol, 20(11), 3427–3431. https://doi.org/10.1111/jocd.14033
Loboda, A., Damulewicz, M., Pyza, E., Jozkowicz, A., & Dulak, J. (2016). Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mole Life Sci, 73(17), 3221–3247. https://doi.org/10.1007/s00018-016-2223-0
Kannan, S., & Jaiswal, A. K. (2006). Low and high dose UVB regulation of transcription factor NF-E2-related factor 2. Cancer Research, 66(17), 8421–8429. https://doi.org/10.1158/0008-5472
Brandner, J. M., Zorn-Kruppa, M., Yoshida, T., Moll, I., Beck, L. A., & De Benedetto, A. (2015). Epidermal tight junctions in health and disease. Tissue Barriers., 3(1–2), e974451. https://doi.org/10.4161/21688370.2014.974451
Yang, M., & Huang, C. Z. (2015). Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World Journal of Gastroenterology, 21(41), 11673–11679. https://doi.org/10.3748/wjg.v21.i41.11673
Cargnello M; Roux pp. (2011). Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mole Biol Rev, 75(1), 50–83. https://doi.org/10.1128/MMBR.00031-10
Li, L., Hwang, E., Ngo, H. T. T., Lin, P., Gao, W., Liu, Y., & Yi, T. H. (2018). Antiphotoaging Effect of Prunus yeonesis blossom extract via inhibition of MAPK/AP-1 and regulation of the TGF-βI/Smad and Nrf2/ARE signaling pathways. Photochemistry and Photobiology, 94(4), 725–732. https://doi.org/10.1111/php.12894
Choi, H. J., Alam, M. B., Baek, M. E., Kwon, Y. G., Lim, J. Y., & Lee, S. H. (2020). Protection against UVB-induced photoaging by nypa fruticans via inhibition of MAPK/AP-1/MMP-1 signaling. Oxidative Medicine and Cellular Longevity, 2020, 2905362. https://doi.org/10.1155/2020/2905362
Ansary, T. M., Hossain, M. R., Kamiya, K., Komine, M., & Ohtsuki, M. (2021). Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Intern J Mole Sci, 22(8), 3974. https://doi.org/10.3390/ijms22083974
Tak, P. P., & Firestein, G. S. (2001). NF-kappaB: a key role in inflammatory diseases. J Clin Inves, 107(1), 7–11. https://doi.org/10.1172/JCI11830
Chen, F., Tang, Y., Sun, Y., Veeraraghavan, V. P., Mohan, S. K., & Cui, C. (2019). 6-shogaol, a active constiuents of ginger prevents UVB radiation mediated inflammation and oxidative stress through modulating NrF2 signaling in human epidermal keratinocytes (HaCaT cells). J Photochem Photobiol Biol, 197, 111518. https://doi.org/10.1016/j.jphotobiol.2019.111518
Hwang, E., Lin, P., Ngo, H. T. T., Ngo, H. T. T., Gao, W., Wang, Y. S., Yu, H. S., & Yi, T. H. (2018). Icariin and icaritin recover UVB-induced photoaging by stimulating Nrf2/ARE and reducing AP-1 and NF-κB signaling pathways: a comparative study on UVB-irradiated human keratinocytes. Photochemical & Photobiological Sciences, 17(10), 1396–1408. https://doi.org/10.1039/c8pp00174j
Zhang, M., Hwang, E., Lin, P., Gao, W., Ngo, H. T. T., & Yi, T. H. (2018). Prunella vulgaris L. Exerts a protective effect against extrinsic aging through NF-κB, MAPKs, AP-1, and TGF-β/Smad signaling pathways in UVB-aged normal human dermal fibroblasts. Rejuvenation Research, 21(4), 313–322. https://doi.org/10.1089/rej.2017.1971
Acknowledgements
This research was kindly supported by Snow White Factory.
Funding
This study was carried out with the support of ´R&D Program for Forest Science Technology (Project No. 2021392B10-2223–0104) provided by Korea Forest Service (Korea Forestry Promotion Institute).
Author information
Authors and Affiliations
Contributions
Conceptualization: THY and SO, methodology: MF, HML, and MSK, software: SZ and DY, validation: MF, HML and SO, formal analysis: MF, HML and JC investigation: THY, MF, HML, and SO, resources: THY, data curation: SZ, MSK, and MJK, writing—SO, and BAD, writing—review and editing: THY and SO, visualization: SZ, BAD, MSK, and MJK, supervision: THY, project administration: THY, All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, M., Lee, HM., Oh, S. et al. Rosa davurica inhibits skin photoaging via regulating MAPK/AP-1, NF-κB, and Nrf2/HO-1 signaling in UVB-irradiated HaCaTs. Photochem Photobiol Sci 21, 2217–2230 (2022). https://doi.org/10.1007/s43630-022-00290-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00290-4