Abstract
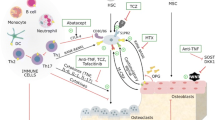
Rheumatoid arthritis (RA) and osteoporosis are two chronic disorders that are often seen together. RA is an autoimmune disorder that causes pain and inflammation in the joints, while osteoporosis is a disorder in which the bones become weak and fragile. Risk factors for bone loss in RA include disease activity, longer disease duration, erosive disease, autoantibody positivity, and joint damage leading to impaired physical activity. Recent research has shown that there is a complex interplay between immune cells, cytokines, and bone remodeling processes in both RA and osteoporosis. The bone remodeling process is regulated by cytokines and immune system signaling pathways, with osteoclasts activated through the RANK/RANKL/OPG pathway and the Wnt/DKK1/sclerostin pathway. Understanding these mechanisms can aid in developing targeted therapies for treatment of osteoporosis in RA patients. Current pharmacological approaches include anti-osteoporotic drugs such as bisphosphonates, denosumab, teriparatide, abaloparatide, raloxifene, and romosozumab. Conventional disease-modifying antirheumatic drugs such as methotrexate and biologicals including TNF inhibitors, IL-6 inhibitors, rituximab, and abatacept lower disease activity in RA and can improve bone metabolism by reducing inflammation but have limited impact on bone mineral density. This review will shed light on the relationship between osteoporosis and rheumatoid arthritis as well as the various factors that influence the onset of osteoporosis in RA patients. We also explore several treatment approaches to effectively managing osteoporosis in RA patients.



Similar content being viewed by others
References
Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C (2021) The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int 41:863–877. https://doi.org/10.1007/s00296-020-04731-0
Weitzmann MN (2013) The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal interface in physiological bone turnover and osteoporosis. Scientifica 2013:e125705. https://doi.org/10.1155/2013/125705
Llorente I, García-Castañeda N, Valero C, González-Álvaro I, Castañeda S (2020) Osteoporosis in rheumatoid arthritis: dangerous liaisons. Front Med 7:601618. https://doi.org/10.3389/fmed.2020.601618
Salari N, Ghasemi H, Mohammadi L, Behzadi MH, Rabieenia E, Shohaimi S et al (2021) The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg 16:609. https://doi.org/10.1186/s13018-021-02772-0
Hauser B, Riches PL, Wilson JF, Horne AE, Ralston SH (2014) Prevalence and clinical prediction of osteoporosis in a contemporary cohort of patients with rheumatoid arthritis. Rheumatology 53:1759–1766. https://doi.org/10.1093/rheumatology/keu162
Jin S, Hsieh E, Peng L, Yu C, Wang Y, Wu C et al (2018) Incidence of fractures among patients with rheumatoid arthritis: a systematic review and meta-analysis. Osteoporos Int 29:1263–1275. https://doi.org/10.1007/s00198-018-4473-1
Phuan-udom R, Lektrakul N, Katchamart W (2018) The association between 10-year fracture risk by FRAX and osteoporotic fractures with disease activity in patients with rheumatoid arthritis. Clin Rheumatol 37:2603–2610. https://doi.org/10.1007/s10067-018-4218-8
Borciani G, Montalbano G, Baldini N, Cerqueni G, Vitale-Brovarone C, Ciapetti G (2020) Co–culture systems of osteoblasts and osteoclasts: Simulating in vitro bone remodeling in regenerative approaches. Acta Biomater 108:22–45. https://doi.org/10.1016/j.actbio.2020.03.043
Raggatt LJ, Partridge NC (2010) Cellular and molecular mechanisms of bone remodeling *. J Biol Chem 285:25103–25108. https://doi.org/10.1074/jbc.R109.041087
Lorenzo J, Horowitz M, Choi Y (2008) Osteoimmunology: interactions of the bone and immune system. Endocr Rev 29:403–440. https://doi.org/10.1210/er.2007-0038
Wysham KD, Baker JF, Shoback DM (2021) Osteoporosis and fractures in rheumatoid arthritis. Curr Opin Rheumatol 33:270. https://doi.org/10.1097/BOR.0000000000000789
Baker R, Narla R, Baker JF, Wysham KD (2022) Risk factors for osteoporosis and fractures in rheumatoid arthritis. Best Pract Res Clin Rheumatol 36:101773. https://doi.org/10.1016/j.berh.2022.101773
Güler-Yüksel M, Hoes JN, Bultink IEM, Lems WF (2018) Glucocorticoids, inflammation and bone. Calcif Tissue Int 102:592–606. https://doi.org/10.1007/s00223-017-0335-7
Fenton CG, Webster JM, Martin CS, Fareed S, Wehmeyer C, Mackie H et al (2019) Therapeutic glucocorticoids prevent bone loss but drive muscle wasting when administered in chronic polyarthritis. Arthritis Res Ther 21:182. https://doi.org/10.1186/s13075-019-1962-3
Chotiyarnwong P, McCloskey EV (2020) Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol 16:437–447. https://doi.org/10.1038/s41574-020-0341-0
Piemontese M, Xiong J, Fujiwara Y, Thostenson JD, O’Brien CA (2016) Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am J Physiol-Endocrinol Metab 311:E587–E593. https://doi.org/10.1152/ajpendo.00219.2016
Delany AM, Durant D, Canalis E (2001) Glucocorticoid suppression of IGF I transcription in osteoblasts. Mol Endocrinol 15:1781–1789. https://doi.org/10.1210/mend.15.10.0704
Sato AY, Richardson D, Cregor M, Davis HM, Au ED, McAndrews K et al (2017) Glucocorticoids induce bone and muscle atrophy by tissue-specific mechanisms upstream of E3 ubiquitin ligases. Endocrinology 158:664–677. https://doi.org/10.1210/en.2016-1779
LoCascio V, Bonucci E, Imbimbo B, Ballanti P, Adami S, Milani S et al (1990) Bone loss in response to long-term glucocorticoid therapy. Bone Miner 8:39–51. https://doi.org/10.1016/0169-6009(91)90139-Q
Wang Y, Zhao R, Gu Z, Dong C, Guo G, Li L (2020) Effects of glucocorticoids on osteoporosis in rheumatoid arthritis: a systematic review and meta-analysis. Osteoporos Int 31:1401–1409. https://doi.org/10.1007/s00198-020-05360-w
Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K et al (2021) 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res 73:924–939. https://doi.org/10.1002/acr.24596
Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. - PMC (n.d.) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1858257/. Accessed 24 Sept 2023
Udagawa N, Koide M, Nakamura M, Nakamichi Y, Yamashita T, Uehara S et al (2021) Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab 39:19–26. https://doi.org/10.1007/s00774-020-01162-6
Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E et al (2001) Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum 44:1003–1012. https://doi.org/10.1002/1529-0131(200105)44:5%3c1003::AID-ANR179%3e3.0.CO;2-#
Danks L, Komatsu N, Guerrini MM, Sawa S, Armaka M, Kollias G et al (2016) RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis 75:1187–1195. https://doi.org/10.1136/annrheumdis-2014-207137
Komatsu N, Takayanagi H (2018) Immune-bone interplay in the structural damage in rheumatoid arthritis. Clin Exp Immunol 194:1–8. https://doi.org/10.1111/cei.13188
Srivastava RK, Dar HY, Mishra PK (2018) Immunoporosis: immunology of osteoporosis—role of T cells. Front Immunol 9:657. https://doi.org/10.3389/fimmu.2018.00657
Zaiss MM, Axmann R, Zwerina J, Polzer K, Gückel E, Skapenko A et al (2007) Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum 56:4104–4112. https://doi.org/10.1002/art.23138
Wang M, Tian T, Yu S, He N, Ma D (2013) Th17 and Treg cells in bone related diseases. Clin Dev Immunol 2013:1–10. https://doi.org/10.1155/2013/203705
Yeo L, Toellner K-M, Salmon M, Filer A, Buckley CD, Raza K et al (2011) Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis 70:2022. https://doi.org/10.1136/ard.2011.153312
Kaneki H, Guo R, Chen D, Yao Z, Schwarz EM, Zhang YE et al (2006) Tumor necrosis factor promotes Runx2 degradation through up-regulation of Smurf1 and Smurf2 in osteoblasts *. J Biol Chem 281:4326–4333. https://doi.org/10.1074/jbc.M509430200
Andreev D, Kachler K, Schett G, Bozec A (2022) Rheumatoid arthritis and osteoimmunology: the adverse impact of a deregulated immune system on bone metabolism. Bone 162:116468. https://doi.org/10.1016/j.bone.2022.116468
Bramlage CP, Häupl T, Kaps C, Ungethüm U, Krenn V, Pruss A et al (2006) Decrease in expression of bone morphogenetic proteins 4 and 5 in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther 8:R58. https://doi.org/10.1186/ar1923
Matzelle M, Shaw A, Baum R, Maeda Y, Li J, Karmakar S et al (2016) Inflammation in arthritis induces expression of BMP3, an inhibitor of bone formation. Scand J Rheumatol 45:379–383. https://doi.org/10.3109/03009742.2015.1126347
Miao C, Yang Y, He X, Li X, Huang C, Huang Y et al (2013) Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal 25:2069–2078. https://doi.org/10.1016/j.cellsig.2013.04.002
Bourhis E, Wang W, Tam C, Hwang J, Zhang Y, Spittler D et al (2011) Wnt antagonists bind through a short peptide to the first β-propeller domain of LRP5/6. Structure 19:1433–1442. https://doi.org/10.1016/j.str.2011.07.005
Ma Y, Zhang X, Wang M, Xia Q, Yang J, Wu M et al (2018) The serum level of Dickkopf-1 in patients with rheumatoid arthritis: a systematic review and meta-analysis. Int Immunopharmacol 59:227–232. https://doi.org/10.1016/j.intimp.2018.04.019
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D et al (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13:156–163. https://doi.org/10.1038/nm1538
(n.d.) Genetic studies on components of the Wnt signalling pathway and the severity of joint destruction in rheumatoid arthritis | Annals of the Rheumatic Diseases. https://ard.bmj.com/content/72/5/769. Accessed 29 Sept 2023)
Sclerostin inhibition reverses systemic, periarticular and local bone loss in arthritis | Annals of the Rheumatic Diseases (n.d.) https://ard.bmj.com/content/72/10/1732. Accessed 29 Sept 2023
Raterman HG, Lems WF (2019) Pharmacological management of osteoporosis in rheumatoid arthritis patients: a review of the literature and practical guide. Drugs Aging 36:1061–1072. https://doi.org/10.1007/s40266-019-00714-4
Sambrook PN, Abeyasekera G, Ansell BM, Foster S, Gumpel JM, Hill PA et al (1985) Calcium absorption in rheumatoid arthritis. Ann Rheum Dis 44:585–588. https://doi.org/10.1136/ard.44.9.585
Hoes JN, Bultink IEM, Lems WF (2015) Management of osteoporosis in rheumatoid arthritis patients. Expert Opin Pharmacother 16:559–571. https://doi.org/10.1517/14656566.2015.997709
Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD et al (2010) Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. The BMJ 341:c3691. https://doi.org/10.1136/bmj.c3691
Hansildaar R, Vedder D, Baniaamam M, Tausche A-K, Gerritsen M, Nurmohamed MT (2021) Cardiovascular risk in inflammatory arthritis: rheumatoid arthritis and gout. Lancet Rheumatol 3:e58-70. https://doi.org/10.1016/S2665-9913(20)30221-6
Kong SH, Jang HN, Kim JH, Kim SW, Shin CS (2022) Effect of vitamin D supplementation on risk of fractures and falls according to dosage and interval: a meta-analysis. Endocrinol Metab 37:344–358. https://doi.org/10.3803/EnM.2021.1374
Charoenngam N (2021) Vitamin D and rheumatic diseases: a review of clinical evidence. Int J Mol Sci 22:10659. https://doi.org/10.3390/ijms221910659
Lin J, Liu J, Davies ML, Chen W (2016) Serum vitamin D level and rheumatoid arthritis disease activity: review and meta-analysis. PLoS ONE 11:e0146351. https://doi.org/10.1371/journal.pone.0146351
Shams-White MM, Chung M, Du M, Fu Z, Insogna KL, Karlsen MC et al (2017) Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation 1,2. Am J Clin Nutr 105:1528–1543. https://doi.org/10.3945/ajcn.116.145110
De Jong Z, Munneke M, Lems WF, Zwinderman AH, Kroon HM, Pauwels EKJ et al (2004) Slowing of bone loss in patients with rheumatoid arthritis by long-term high-intensity exercise: results of a randomized, controlled trial. Arthritis Rheum 50:1066–1076. https://doi.org/10.1002/art.20117
Bennett JL, Pratt AG, Dodds R, Sayer AA, Isaacs JD (2023) Rheumatoid sarcopenia: loss of skeletal muscle strength and mass in rheumatoid arthritis. Nat Rev Rheumatol 19:239–251. https://doi.org/10.1038/s41584-023-00921-9
Ngeuleu A, Allali F, Medrare L, Madhi A, Rkain H, Hajjaj-Hassouni N (2017) Sarcopenia in rheumatoid arthritis: prevalence, influence of disease activity and associated factors. Rheumatol Int 37:1015–1020. https://doi.org/10.1007/s00296-017-3665-x
Tada M, Yamada Y, Mandai K, Matsumoto Y, Hidaka N (2021) Osteosarcopenia synergistically increases the risk of falls in patients with rheumatoid arthritis. Osteoporos Sarcopenia 7:140–145. https://doi.org/10.1016/j.afos.2021.11.002
Torii M, Itaya T, Minamino H, Katsushima M, Fujita Y, Tanaka H et al (2023) Management of sarcopenia in patients with rheumatoid arthritis. Mod Rheumatol 33:435–440. https://doi.org/10.1093/mr/roac095
Cremers S, Drake MT, Ebetino FH, Bilezikian JP, Russell RGG (2019) Pharmacology of bisphosphonates. Br J Clin Pharmacol 85:1052–1062. https://doi.org/10.1111/bcp.13867
Santora AC, Sharma A (2020) Bisphosphonates: mechanisms of action and role in osteoporosis therapy. In: Leder BZ, Wein MN (eds.) Osteoporos Pathophysiol Clin Manag, Cham: Springer International Publishing, pp 277–307 https://doi.org/10.1007/978-3-319-69287-6_14
Eggelmeijer F, Papapoulos SE, van Paassen HC, Dijkmans BAC, Valkema R, Westedt ML et al (1996) Increased bone mass with pamidronate treatment in rheumatoid arthritis. Results of a three-year randomized, double-blind trial. Arthritis Rheum 39:396–402. https://doi.org/10.1002/art.1780390307
Fujieda Y, Horita T, Nishimoto N, Tanimura K, Amasaki Y, Kasahara H et al (2021) Efficacy and safety of sodium RISedronate for glucocorticoid-induced OsTeoporosis with rheumaTOid arthritis (RISOTTO study): a multicentre, double-blind, randomized, placebo-controlled trial. Mod Rheumatol 31:593–599. https://doi.org/10.1080/14397595.2020.1812835
Kumagai K, Harigane K, Kusayama Y, Tezuka T, Choe H, Inaba Y et al (2018) Effects of once-monthly minodronate versus risedronate in osteoporosis patients with rheumatoid arthritis: a 12-month randomized head-to-head comparison. Osteoporos Int 29:1637–1642. https://doi.org/10.1007/s00198-018-4494-9
Peris P, Monegal A, Guañabens N (2021) Bisphosphonates in inflammatory rheumatic diseases. Bone 146:115887. https://doi.org/10.1016/j.bone.2021.115887
Sansoni P, Passeri G, Fagnoni F, Mohagheghpour N, Snelli G, Brianti V et al (2009) Inhibition of antigen-presenting cell function by alendronate in vitro. J Bone Miner Res 10:1719–1725. https://doi.org/10.1002/jbmr.5650101115
Valleala H, Laasonen L, Koivula M-K, Mandelin J, Friman C, Risteli J et al (2003) Two year randomized controlled trial of etidronate in rheumatoid arthritis: changes in serum aminoterminal telopeptides correlate with radiographic progression of disease. J Rheumatol 30:468–473
Jarrett SJ, Conaghan PG, Sloan VS, Papanastasiou P, Ortmann C, O’Connor PJ et al (2006) Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum 54:1410–1414. https://doi.org/10.1002/art.21824
Lewiecki EM (2020) Denosumab: mechanisms and therapeutic effects in the treatment of osteoporosis. In: Leder BZ, Wein MN (eds.) Osteoporos Pathophysiol Clin Manag, Cham: Springer International Publishing, p 309–22. https://doi.org/10.1007/978-3-319-69287-6_15
Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T et al (2000) Involvement of receptor activator of nuclear factor κB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 43:259–269. https://doi.org/10.1002/1529-0131(200002)43:2%3c259::AID-ANR4%3e3.0.CO;2-W
Tanaka S, Tanaka Y (2021) RANKL as a therapeutic target of rheumatoid arthritis. J Bone Miner Metab 39:106–112. https://doi.org/10.1007/s00774-020-01159-1
Yamaguchi Y, Morita T, Kumanogoh A (2020) The therapeutic efficacy of denosumab for the loss of bone mineral density in glucocorticoid-induced osteoporosis: a meta-analysis. Rheumatol Adv Pract 4. https://doi.org/10.1093/rap/rkaa008
Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT et al (2008) Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum 58:1299–1309. https://doi.org/10.1002/art.23417
Takeuchi T, Tanaka Y, Soen S, Yamanaka H, Yoneda T, Tanaka S et al (2019) Effects of the anti-RANKL antibody denosumab on joint structural damage in patients with rheumatoid arthritis treated with conventional synthetic disease-modifying antirheumatic drugs (DESIRABLE study): a randomised, double-blind, placebo-controlled phase 3 trial. Ann Rheum Dis 78:899–907. https://doi.org/10.1136/annrheumdis-2018-214827
Hasegawa T, Kaneko Y, Izumi K, Takeuchi T (2017) Efficacy of denosumab combined with bDMARDs on radiographic progression in rheumatoid arthritis. Joint Bone Spine 84:379–380. https://doi.org/10.1016/j.jbspin.2016.05.010
Vall H, Teriparatide PM (2023) StatPearls. StatPearls Publishing, Treasure Island (FL)
Liu Z-M, Zhang M, Zong Y, Zhang D, Shen Z-B, Guan X-Q et al (2022) The efficiency and safety of alendronate versus teriparatide for treatment glucocorticoid-induced osteoporosis: a meta-analysis and systematic review of randomized controlled trials. PLoS ONE 17:e0267706. https://doi.org/10.1371/journal.pone.0267706
Ebina K, Hirao M, Hashimoto J, Hagihara K, Kashii M, Kitaguchi K et al (2018) Assessment of the effects of switching oral bisphosphonates to denosumab or daily teriparatide in patients with rheumatoid arthritis. J Bone Miner Metab 36:478–487. https://doi.org/10.1007/s00774-017-0861-4
Langdahl BL, Silverman S, Fujiwara S, Saag K, Napoli N, Soen S et al (2018) Real-world effectiveness of teriparatide on fracture reduction in patients with osteoporosis and comorbidities or risk factors for fractures: integrated analysis of 4 prospective observational studies. Bone 116:58–66. https://doi.org/10.1016/j.bone.2018.07.013
Solomon DH, Kay J, Duryea J, Lu B, Bolster MB, Yood RA et al (2017) Effects of teriparatide on joint erosions in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol 69:1741–1750. https://doi.org/10.1002/art.40156
Akel M, Abaloparatide PM (2023) StatPearls. StatPearls Publishing, Treasure Island (FL)
Sleeman A, Clements JN (2019) Abaloparatide: a new pharmacological option for osteoporosis. Am J Health Syst Pharm 76:130–135. https://doi.org/10.1093/ajhp/zxy022
Quintanilla Rodriguez BS, Correa R (2023) Raloxifene. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL. https://www.ncbi.nlm.nih.gov/books/NBK544233/
Humphrey MB, Russell L, Danila MI, Fink HA, Guyatt G, Cannon M et al (2023) 2022 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis & Rheumatology 75:2088–2102. https://doi.org/10.1002/art.42646
Krupa K, Parmar M, Delo LF (2023) Romosozumab. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL. https://www.ncbi.nlm.nih.gov/books/NBK585139/
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A et al (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370:412–420. https://doi.org/10.1056/NEJMoa1305224
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T et al (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377:1417–1427. https://doi.org/10.1056/NEJMoa1708322
Lewiecki EM, Blicharski T, Goemaere S, Lippuner K, Meisner PD, Miller PD et al (2018) A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab 103:3183–3193. https://doi.org/10.1210/jc.2017-02163
Mok CC (2023) Romosozumab versus denosumab for osteoporosis in long-term glucocorticoid users: an open randomized controlled trial. Identifier: NCT04091243. clinicaltrials.gov. https://clinicaltrials.gov/study/NCT04091243. Accessed 16 Mar 2024
Mochizuki T, Yano K, Ikari K, Hiroshima R, Okazaki K (2023) Comparison of romosozumab versus denosumab treatment on bone mineral density after 1 year in rheumatoid arthritis patients with severe osteoporosis: a randomized clinical pilot study. Mod Rheumatol 33:490–495. https://doi.org/10.1093/mr/roac059
di Munno O, Mazzantini M, Sinigaglia L, Bianchi G, Minisola G, Muratore M et al (2004) Effect of low dose methotrexate on bone density in women with rheumatoid arthritis: results from a multicenter cross-sectional study. J Rheumatol 31:1305–1309
Kwon OC, Oh JS, Hong S, Lee C-K, Yoo B, Kim Y-G (2019) Conventional synthetic disease-modifying antirheumatic drugs and bone mineral density in rheumatoid arthritis patients with osteoporosis: possible beneficial effect of leflunomide. Clin Exp Rheumatol 37:813–819
Zerbini CAF, Clark P, Mendez-Sanchez L, Pereira RMR, Messina OD, Uña CR et al (2017) Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 28:429–46. https://doi.org/10.1007/s00198-016-3769-2
Marotte H, Pallot-Prades B, Grange L, Gaudin P, Alexandre C, Miossec P (2007) A 1-year case-control study in patients with rheumatoid arthritis indicates prevention of loss of bone mineral density in both responders and nonresponders to infliximab. Arthritis Res Ther 9:R61. https://doi.org/10.1186/ar2219
Vis M, Gj Wolbink, Lodder MC, Kostense PJ, van de Stadt RJ, de Koning MHMT et al (2003) Early changes in bone metabolism in rheumatoid arthritis patients treated with infliximab. Arthritis Rheum 48:2996–7. https://doi.org/10.1002/art.11292
Kim SY, Schneeweiss S, Liu J, Solomon DH (2012) Effects of disease-modifying antirheumatic drugs on nonvertebral fracture risk in rheumatoid arthritis: a population-based cohort study. J Bone Miner Res 27:789–796. https://doi.org/10.1002/jbmr.1489
Coulson KA, Reed G, Gilliam BE, Kremer JM, Pepmueller PH (2009) Factors influencing fracture risk, T score, and management of osteoporosis in patients with rheumatoid arthritis in the Consortium of Rheumatology Researchers of North America (CORRONA) Registry. JCR J Clin Rheumatol 15:155. https://doi.org/10.1097/RHU.0b013e3181a5679d
Axmann R, Böhm C, Krönke G, Zwerina J, Smolen J, Schett G (2009) Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum 60:2747–2756. https://doi.org/10.1002/art.24781
Garnero P, Thompson E, Woodworth T, Smolen JS (2010) Rapid and sustained improvement in bone and cartilage turnover markers with the anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a substudy of the multicenter double-blind, placebo-controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum 62:33–43. https://doi.org/10.1002/art.25053
Abu-Shakra M, Zisman D, Balbir-Gurman A, Amital H, Levy Y, Langevitz P et al (2018) Effect of tocilizumab on fatigue and bone mineral density in patients with rheumatoid arthritis. Isr Med Assoc J IMAJ 20:239–244
Suzuki T, Nakamura Y, Kato H (2018) Effects of denosumab on bone metabolism and bone mineral density with anti-TNF inhibitors, tocilizumab, or abatacept in osteoporosis with rheumatoid arthritis. Ther Clin Risk Manag 14:453–459. https://doi.org/10.2147/TCRM.S156350
Orsolini G, Fassio A, Rossini M, Adami G, Giollo A, Caimmi C et al (2019) Effects of biological and targeted synthetic DMARDs on bone loss in rheumatoid arthritis. Pharmacol Res 147:104354. https://doi.org/10.1016/j.phrs.2019.104354
Boumans MJH, Thurlings RM, Yeo L, Scheel-Toellner D, Vos K, Gerlag DM et al (2012) Rituximab abrogates joint destruction in rheumatoid arthritis by inhibiting osteoclastogenesis. Ann Rheum Dis 71:108–113. https://doi.org/10.1136/annrheumdis-2011-200198
Onal M, Xiong J, Chen X, Thostenson JD, Almeida M, Manolagas SC et al (2012) Receptor activator of nuclear factor κB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem 287:29851–29860. https://doi.org/10.1074/jbc.M112.377945
Wheater G, Elshahaly M, Naraghi K, Tuck SP, Datta HK, van Laar JM (2018) Changes in bone density and bone turnover in patients with rheumatoid arthritis treated with rituximab, results from an exploratory, prospective study. PLoS ONE 13:e0201527. https://doi.org/10.1371/journal.pone.0201527
Kim Y, Kim G-T (2023) Positive effects of biologics on osteoporosis in rheumatoid arthritis. J Rheum Dis 30:3–17. https://doi.org/10.4078/jrd.22.0046
Tada M, Inui K, Sugioka Y, Mamoto K, Okano T, Koike T (2018) Abatacept might increase bone mineral density at femoral neck for patients with rheumatoid arthritis in clinical practice: AIRTIGHT study. Rheumatol Int 38:777–784. https://doi.org/10.1007/s00296-017-3922-z
Chen M-H, Yu S-F, Chen J-F, Chen W-S, Liou T-L, Chou C-T et al (2021) Different effects of biologics on systemic bone loss protection in rheumatoid arthritis: an interim analysis of a three-year longitudinal cohort study. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.783030
Adam S, Simon N, Steffen U, Andes FT, Scholtysek C, Müller DIH et al (2020) JAK inhibition increases bone mass in steady-state conditions and ameliorates pathological bone loss by stimulating osteoblast function. Sci Transl Med 12:eaay4447. https://doi.org/10.1126/scitranslmed.aay4447
Komagamine M, Komatsu N, Ling R, Okamoto K, Tianshu S, Matsuda K et al (2023) Effect of JAK inhibitors on the three forms of bone damage in autoimmune arthritis: joint erosion, periarticular osteopenia, and systemic bone loss. Inflamm Regen 43:44. https://doi.org/10.1186/s41232-023-00293-3
Sugahara S, Hanaoka K, Emori T, Takeshita N, Fujii Y, Nakano M et al (2022) Peficitinib improves bone fragility by recovering bone turnover imbalance in arthritic mice. J Pharmacol Sci 148:134–141. https://doi.org/10.1016/j.jphs.2021.10.006
Hamar A, Szekanecz Z, Pusztai A, Czókolyová M, Végh E, Pethő Z et al (2021) Effects of one-year tofacitinib therapy on bone metabolism in rheumatoid arthritis. Osteoporos Int 32:1621–1629. https://doi.org/10.1007/s00198-021-05871-0
Murakami K, Kobayashi Y, Uehara S, Suzuki T, Koide M, Yamashita T et al (2017) A Jak1/2 inhibitor, baricitinib, inhibits osteoclastogenesis by suppressing RANKL expression in osteoblasts in vitro. PLoS ONE 12:e0181126. https://doi.org/10.1371/journal.pone.0181126
Acknowledgements
This study was carried out under the Indian Council of Medical Research-Short Term Studentship (ICMR-STS) 2022 program. We acknowledge the support of ICMR-New Delhi and All India Institute of Medical Sciences, Bathinda. We thank NG and NK for writing the manuscript; AA and KK for making all necessary changes and revising the manuscript; AK for editing, revision, and proof reading; and KK and AK for conceptualizing and execution of the study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, N., Kanwar, N., Arora, A. et al. The interplay of rheumatoid arthritis and osteoporosis: exploring the pathogenesis and pharmacological approaches. Clin Rheumatol 43, 1421–1433 (2024). https://doi.org/10.1007/s10067-024-06932-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-024-06932-5