Abstract
Introduction
Sacroiliac bone marrow edema is an important factor in the diagnosis and management of axial spondyloarthritis (axSpA). The aim of this meta-analysis is to assess the effect of the different bDMARDs and tsDMARDs on the SPARCC score at 12–16 and 48–52 weeks.
Methods
A systematic review, performed on PubMed (including Medline), Cochrane (CENTRAL) and DOAJ databases, included randomized controlled studies evaluating the sacroiliac joint (SIJ) SPARCC score at 12–16 or 48–52 weeks in patients with axSpA meeting the ASAS 2009 criteria or the modified New York criteria. We included studies evaluating the effects of the different treatments on the SPARCC score of SIJ in axial spondyloarthritis in comparison to a control group.
Results
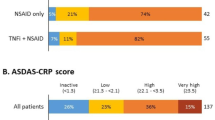
Eighteen studies were included in the meta-analysis. Nine studies evaluated the effect of TNFα inhibitors (TNFi), three for IL-17 inhibitors, and four for JAK inhibitors. At 12 and 16 weeks, SIJ SPARCC score was significantly improved by TNFi (WMD: − 3.29 [95% CI − 4.25; − 2, 34]), by IL-17 inhibitors (WMD: − 4.66 [95% CI − 6.22; − 3.09]), and by JAK inhibitors (JAKi) (WMD: − 3.06 [95% CI − 3.24; − 2.89]). There was no difference between the molecule subgroups. At 48–52 weeks, TNFα inhibitors reduced more SIJ SPARCC, but not significantly (WMD: − 2.26 [95% CI − 4.94; 0.42]), than placebo groups who began a TNFi treatment with delay.
Conclusion
Our meta-analysis shows a comparable improvement of the SIJ SPARCC score regarding TNFi, JAKi, and IL-17 inhibitors at three months and suggests the presence of an opportunity window.
Key Points • Anti-TNF Ab, anti-IL17 Ab, and JAK inhibitor treatments reduce the sacroiliac joint SPARCC scores. • There is no difference between the different treatments in the reduction of the sacroiliac joint SPARCC score after 3 months in axial spondyloarthritis. |




Similar content being viewed by others
References
Sieper J, Poddubnyy D (2017) Axial spondyloarthritis. Lancet Lond Engl 390:73–84
Rudwaleit M, van der Heijde D, Landewé R et al (2009) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68:777–783
van der Heijde D, Lie E, Kvien TK et al (2009) Assessment of SpondyloArthritis international Society (ASAS). ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 68:1811–1818
van der Heijde D, Braun J, Deodhar A et al (2019) Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology 58:388–400
Aouad K, Ziade N, Baraliakos X (2020) Structural progression in axial spondyloarthritis. Joint Bone Spine 87:131–136
Wendling D, Hecquet S, Fogel O et al (2022) French Society for Rheumatology (SFR) recommendations on the everyday management of patients with spondyloarthritis, including psoriatic arthritis. Joint Bone Spine 89:105344
Baraliakos X, Boehm H, Bahrami R et al (2019) What constitutes the fat signal detected by MRI in the spine of patients with ankylosing spondylitis? A prospective study based on biopsies obtained during planned spinal osteotomy to correct hyperkyphosis or spinal stenosis. Ann Rheum Dis 78:1220–1225
Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ (2014) Fat metaplasia and backfill are key intermediaries in the development of sacroiliac joint ankylosis in patients with ankylosing spondylitis. Arthritis Rheum 66:2958–2967
van der Heijde D, Sieper J, Maksymowych WP et al (2018) Clinical and MRI remission in patients with nonradiographic axial spondyloarthritis who received long-term open-label adalimumab treatment: 3-year results of the ABILITY-1 trial. Arthritis Res Ther 20:61
Herrada I, Devilliers H, Fayolle C et al (2021) Diagnostic performance of sacroiliac and spinal MRI for the diagnosis of non-radiographic axial spondyloarthritis in patients with inflammatory back pain. Joint Bone Spine 88:105106
Maksymowych WP, Lambert RG, Østergaard M et al (2019) MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis 78:1550–1558
Maksymowych WP, Inman RD, Salonen D et al (2005) Spondyloarthritis research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 53:703–709
Braun J, Baraliakos X, Golder W et al (2003) Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum 2003(48):1126–1136
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368
Jadad AR, Moore RA, Carroll D et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Lambert RGW, Salonen D, Rahman P et al (2007) Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 56:4005–4014
Hu Z, Xu M, Li Q et al (2012) Adalimumab significantly reduces inflammation and serum DKK-1 level but increases fatty deposition in lumbar spine in active ankylosing spondylitis. Int J Rheum Dis 15:358–365
Sieper J, van der Heijde D, Dougados M et al (2013) Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis 72:815–822
Sieper J, van der Heijde D, Dougados M et al (2015) A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheum 67:2702–2712
Pedersen SJ, Poddubnyy D, Sørensen IJ et al (2016) Course of magnetic resonance imaging-detected inflammation and structural lesions in the sacroiliac joints of patients in the randomized, double-blind, placebo-controlled Danish multicenter study of adalimumab in spondyloarthritis, as assessed by the Berlin and Spondyloarthritis Research Consortium of Canada Methods. Arthritis Rheum 68:418–429
Hededal P, Østergaard M, Sørensen IJ et al (2018) Development and validation of MRI Sacroiliac joint scoring methods for the semiaxial scan plane corresponding to the Berlin and SPARCC MRI scoring methods, and of a new global MRI sacroiliac joint method. J Rheumatol 45:70–77
Dougados M, van der Heijde D, Sieper J et al (2017) Effects of long-term etanercept treatment on clinical outcomes and objective signs of inflammation in early nonradiographic axial spondyloarthritis: 104-week results from a randomized, placebo-controlled study. Arthritis Care Res 69:1590–1598
Braun J, Baraliakos X, Hermann K-G et al (2017) Effect of certolizumab pegol over 96 weeks of treatment on inflammation of the spine and sacroiliac joints, as measured by MRI, and the association between clinical and MRI outcomes in patients with axial spondyloarthritis. RMD Open 3:e000430
Deodhar A, Gensler LS, Kay J et al (2019) A fifty-two-week, randomized, placebo-controlled trial of certolizumab pegol in nonradiographic axial spondyloarthritis. Arthritis Rheum 71:1101–1111
Mok CC, Li OC, Chan KL, Ho LY, Hui PK (2015) Effect of golimumab and pamidronate on clinical efficacy and MRI inflammation in axial spondyloarthritis: a 48-week open randomized trial. Scand J Rheumatol 44:480–486
Maksymowych WP, Dougados M, van der Heijde D et al (2016) Clinical and MRI responses to etanercept in early non-radiographic axial spondyloarthritis: 48-week results from the EMBARK study. Ann Rheum Dis 75:1328–1335
Deodhar A, van der Heijde D, Gensler LS et al (2020) Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 395:53–64
Van der Heijde D, Cheng-Chung Wei J, Dougados M et al (2018) Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 392:2441–2451
Van der Heijde D, Deodhar A, Baraliakos X et al (2023) Efficacy and safety of bimekizumab in axial spondyloarthritis: results of two parallel phase 3 randomised controlled trials. Ann Rheum Dis 82:515–526
Van der Heijde D, Deodhar A, Wei JC et al (2017) Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 76:1340–1347
Van der Heijde D, Baraliakos X, Gensler LS et al (2018) Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet 392:2378–2387
Van der Heijde D, Baraliakos X, Sieper J et al (2022) Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. Ann Rheum Dis 81:1515–1523
Deodhar A, Van den Bosch F, Poddubnyy D et al (2022) Upadacitinib for the treatment of active non-radiographic axial spondyloarthritis (SELECT-AXIS 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 400:369–379
Huang Y, Chen Y, Liu T, Lin S, Yin G, Xie Q (2020) Impact of tumor necrosis factor α inhibitors on MRI inflammation in axial spondyloarthritis assessed by Spondyloarthritis Research Consortium Canada score: a meta-analysis. PLoS One 15:e0244788
Lee YH, Song GG (2020) Janus kinase inhibitors for treating active ankylosing spondylitis: a meta-analysis of randomized controlled trials. Z Rheumatol 81:71–76
Mauro D, Gandolfo S, Tirri E, Schett G, Maksymowych WP, Ciccia F (2023) The bone marrow side of axial spondyloarthritis. Nat Rev Rheumatol 19:519–532
Baraliakos X, Østergaard M, Gensler LS, SURPASS Study Group et al (2020) Comparison of the effects of secukinumab and adalimumab biosimilar on radiographic progression in patients with ankylosing spondylitis: design of a randomized, phase IIIb study (SURPASS). Clin Drug Investig. 40:269–278
Robinson PC, Brown MA (2014) The window of opportunity: a relevant concept for axial spondyloarthritis. Arthritis Res Ther 16:109
Maksymowych WP, Morency N, Conner-Spady B, Lambert RG (2013) Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis 72:23–8
Van der Heijde D, Baraliakos X, Hermann K-GA et al (2018) Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann Rheum Dis 77:699–705
Deodhar A, Blanco R, Dokoupilová E et al (2021) Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheum 73:110–20
Michelena X, López-Medina C, Marzo-Ortega H (2020) Non-radiographic versus radiographic axSpA: what’s in a name? Rheumatology 59(Suppl4):iv18-24
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aude Hansmaennel is the first author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hansmaennel, A., Fakih, O., Gerazime, A. et al. Effects of disease-modifying anti-rheumatic drugs on sacroiliac MRI score in axial spondyloarthritis: a systematic review and meta-analysis. Clin Rheumatol 43, 1045–1052 (2024). https://doi.org/10.1007/s10067-023-06849-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06849-5