Abstract
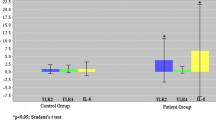
Although tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin-17 (IL-17) play important roles in RA, their relative expression and possible correlation in synovial tissues are not well understood. In this study, mRNA expression levels of IFN-γ, IL-17, and TNF-α were investigated in individual patients with RA and the correlations between pairs of these three pro-inflammatory cytokines were analyzed. Synovial tissues were obtained during arthroplasties from 24 joints of 24 RA patients. After harvesting synovial tissues, total RNA was isolated then quantitative real-time polymerase chain reaction (qRT-PCR) for IFN-γ, IL-17, and TNF-α was performed. Correlation of expression levels between them was also analyzed. Expression levels of TNF-α, IFN-γ, and IL-17 in patients receiving TNF inhibitors (TNFi) and those treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) alone were also compared between groups. Based on relative expression levels of the three pro-inflammatory cytokines, patients were classified into three major types; an IFN-γ plus TNF-α-dominant type, an IL-17-dominant type, and the other type. TNF-α expression levels were correlated with IFN-γ. In addition, there was a negative correlation between TNF-α and IL-17, and IFN-γ and IL-17. Median relative expression levels of TNF-α have no significant difference between the TNFi and the csDMARDs groups. In the rheumatoid synovial tissues, expression levels of TNF-α were modulated in parallel with IFN-γ, and TNF-α and IL-17, or IFN-γ and IL-17 did not co-express at high levels. This characteristic expression pattern of the three pro-inflammatory cytokines may be clinically useful information in the current cytokine-targeted treatment with biological DMARDs for RA.



Similar content being viewed by others
References
Arend WP, Dayer JM (1995) Inhibition of the production and effects of interleukin-1 and tumor necrosis factor in rheumatoid arthritis. Arthritis Rheum 38:151–160
Murphy G, Nagase H (2008) Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol 4:128–135
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423:356–361
Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L et al (1999) Human interleukin-17: a T cell-derived pro-inflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum 42:963–970
Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S et al (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103:1345–1352
Raza K, Falciani F, Curnow SJ, Ross EJ, Lee CY, Akbar AN et al (2005) Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther 7:R784–R795
Kato H, Endres J, Fox DA (2013) The roles of IFN-γ versus IL-17 in pathogenic effects of human Th17 cells on synovial fibroblasts. Mod Rheumatol 23:1140–1150
Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S (2009) Type 17 T helper cells: origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol 5:325–331
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Kanbe K, Hara R, Chiba J, Inoue Y, Taguchi M, Tanaka Y (2014) Application of a new immunohistology scoring system (IH score): analysis of TNF-α in synovium related to disease activity score in infliximab-treated patients with rheumatoid arthritis. Mod Rheumatol 24:910–914
Nguyen VT, Benveniste EN (2002) Critical role of tumor necrosis factor-alpha and NF-kappa B in interferon-gamma -induced CD40 expression in microglia/macrophages. J Biol Chem 277:13796–13803
Miossec P, Kolls JK (2012) Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 11:763–776
Furst DE, Emery P (2014) Rheumatoid arthritis pathophysiology: update on emerging cytokine and cytokine-associated cell targets. Rheumatology (Oxford) 53:1560–1569
Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y et al (2006) Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203:2673–2682
Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K et al (2000) T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 408:600–605
Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL (2011) Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapy. Arthritis Res Ther 13:R126
Alzabin S, Abraham SM, Taher TE, Palfreeman A, Hull D, McNamee K et al (2012) Incomplete response of inflammatory arthritis to TNFα blockade is associated with the Th17 pathway. Ann Rheum Dis 71:1741–1748
Genovese MC, Greenwald M, Cho CS, Berman A, Jin L, Cameron GS et al (2014) A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol 66:1693–1704
Lubberts E (2008) IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine 41:84–91
Bhatia A, Kast RE (2007) Tumor necrosis factor (TNF) can paradoxically increase on etanercept treatment, occasionally contributing to TNF-mediated disease. J Rheumatol 34:447–449
Kast RE, Altschuler EL (2008) Both etanercept and infliximab can elevate tumor necrosis factor (TNF)-alpha and be the cause of treatment related new onset disease: the need to measure circulating TNF-alpha. J Rheumatol 35:1679
Edrees AF, Misra SN, Abdou NI (2005) Anti-tumor necrosis factor (TNF) therapy in rheumatoid arthritis: correlation of TNF-alpha serum level with clinical response and benefit from changing dose or frequency of infliximab infusions. Clin Exp Rheumatol 23:469–474
Moghadam MG, Vonkeman HE, Ten Klooster PM, Tekstra J, van Schaardenburg D, Starmans-Kool M et al (2016) Dutch National POET Collaboration. Stopping tumor necrosis factor-inhibitors in patients with established rheumatoid arthritis in remission or stable low disease activity: a pragmatic randomized multicenter open-label controlled trial. Arthritis Rheumatol. [Epub ahead of print]
Acknowledgments
This work was supported in part by Grant-in-Aid (23592233 to A. N., 23592234 to M. So.) from the Ministry of Education, Science, Technology, Sports and Culture of Japan, the Futaba Electronics Memorial Foundation (A. N.), the Takeda Science Foundation (A. N.), the Research Promotion Grant from Toho University Graduate School of Medicine (No. 12-03 to A. N.), and the Hamaguchi Foundation for the Advancement of Biochemistry (A. N.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Written informed consent was obtained from all the patients prior to surgery. The study protocol was approved by the Ethical Committee for Clinical Research at Toho University (Approved No. 2013-009).
Disclosures
None.
Rights and permissions
About this article
Cite this article
Nakajima, A., Aoki, Y., Sonobe, M. et al. Relative expression and correlation of tumor necrosis factor-α, interferon-γ, and interleukin-17 in the rheumatoid synovium. Clin Rheumatol 35, 1691–1697 (2016). https://doi.org/10.1007/s10067-016-3249-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3249-2