Abstract
Online hemodiafiltration (OHDF) for renal replacement therapy has two modes: pre- (pre-OHDF) and post-dilution OHDF (post-OHDF). To elucidate the precise differences between the two modes, a clinical study was performed using the same polysulfone hemodiafilters in the same patients. Eight patients were treated with ABH™-22PA for 6 weeks: 3 weeks of pre-OHDF (with substitution volumes of 24, 36, and 48 L) and 3 weeks of post-OHDF (6, 8, and 10 L). The reduction ratios of urea, uric acid (UA), creatinine (CRE), inorganic phosphorus (iP), beta-2-microglobulin (β2-MG), and alpha-1-microglobulin (α1-MG) were evaluated. The removal amounts of β2-MG, α1-MG, and albumin were also evaluated by analyzing the spent dialysis fluids. The types and numbers of adverse events (AEs) and device malfunctions were recorded. The reduction ratios of urea, UA, CRE, iP, and β2-MG were comparable among all conditions, while that of α1-MG tended to be slightly higher in post-OHDF than in pre-OHDF. The removal amounts of α1-MG and albumin in pre-OHDF and post-OHDF were significantly greater with the maximum substitution volume than with the minimum volume. However, the selective removal indices, which were obtained by dividing the amount of α1-MG removed by the albumin level, tended to be slightly higher in pre- than in post-OHDF. No device-related AEs or device malfunctions occurred in either mode. No significant differences in inflammatory responses, evaluated by high-sensitivity C-reactive protein and interleukin-6, were observed. This study provides removal performance and safety data regarding the application of ABH-22PA for pre- and post-OHDF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
End-stage kidney disease (ESKD) is a major public health concern, and over 2 million people worldwide require renal replacement therapy to sustain life [1]. The three therapeutic options for renal replacement therapy are hemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation. Patients with ESKD on HD or PD have a shorter life expectancy and lower quality of life (QOL) than those undergoing kidney transplantation. Over the past decades, online hemodiafiltration (OHDF) has become available and is defined as a combination of diffuse and convective solute transport using a high-flux membrane [2]. OHDF has been developed to remove middle molecules [3], which are also known as low-molecular-weight proteins (LMWPs) [4], more effectively than conventional HD. Aggressively removing LMWPs could lead to better survival rates in patients undergoing maintenance dialysis [4]. By increasing the reduction ratio of the alpha-1-microglobulin (α1-MG), one such middle molecule, an improvement in clinical manifestations and patients’ QOL can be expected [5]. The benefits of OHDF on all-cause mortality over conventional HD have been reported in three large randomized controlled trials [6,7,8]. However, a recent report showed that survival was equivalent between OHDF and super high-flux HD patients with similar albumin leakage [9].
The dilution modes for OHDF are pre-dilution (pre-OHDF) and post-dilution (post-OHDF). Post-OHDF is used in Europe and many other countries worldwide, whereas pre-OHDF is mainly used in Japan [4]. Pre-OHDF is considered advantageous because hemoconcentration is unlikely inside the filter; therefore, higher substitution volumes can be used even in patients with relatively low blood flow rates [10]. Due to hemoconcentration inside the filter, the shear stress on the blood cells activates platelets in post-OHDF [11]. Moreover, pre-OHDF may have more favorable effects on blood cells than post-OHDF because interleukin-6 (IL-6) and intercellular adhesion molecule (ICAM)-1 have been reported to be decreased only in pre-OHDF [12].
Post-OHDF has been reported to result in significantly lower all-cause mortality than conventional HD [13, 14]. Pre-OHDF was also demonstrated to have a significantly lower all-cause mortality than conventional HD in two retrospective studies [10, 14, 15]. In contrast, all-cause mortality did not significantly differ between pre- and post-OHDF [14]. Moreover, pre- and post-OHDF are known to lower the mortality in patients who receive a higher substitution volume [13, 15], while another report stated that mortality of pre-OHDF was significantly correlated with albumin leakage rather than substitution volume [9]. Therefore, differences in removal performance may affect mortality. Furthermore, if the same hemodiafilters are used in the same patients, the removal performance would still differ depending on the dilution mode [5]. Some studies focusing on evaluating the removal performances in OHDF have been reported [16,17,18]. However, these studies have not assessed the differences in removal performance between pre- and post-OHDF with various substitution volumes in the same hemodiafilters and patients.
In this study, the removal performance of pre- and post-OHDF under various substitution volumes was evaluated using the same hemodiafilter in the same patients. In addition, the effects of the different dilution modes on the inflammatory responses were compared.
Materials and methods
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki of the World Medical Association. All subjects enrolled in this research provided their written informed consent, which was approved by the clinical research review board certified by the Ministry of Health, Labour and Welfare of Japan, an institutional committee on human and/or animal research that confirmed the study protocol was acceptable. This study is registered at the Japan Registry of Clinical Trials (jRCT) with the registration number jRCTs062190020.
Patients
Eight male patients were enrolled at the start of the study. The inclusion criteria were as follows: stable maintenance OHDF therapy for at least one month; capable of obtaining QB ≥ 280 mL/min; use of hemodiafiltration with a surface area ≥ 2.2 m2; on OHDF for > 4 h per treatment; capable of participating in the study as an outpatient; capable of understanding the informed consent form; and age between 20 and 85 years. The exclusion criteria were as follows: patients who required blood purification therapy other than OHDF (such as PD) during the study period; those who had a medical history of anaphylaxis caused by polysulfone or polyvinylpyrrolidone (PVP); those who had participated in other clinical trials during the study period that could influence the results of this study; and disqualification from participation according to the opinion of the principal investigator.
Study design
This was a prospective, open-label, non-randomized, single-arm study. Each patient received OHDF therapy using ABH-22PA (surface area: 2.2 m2 polysulfone membrane, gamma irradiation sterilization; Asahi Kasei Medical Co., Ltd. Tokyo, Japan) for 6 weeks. Each patient was administered pre-OHDF for the first 3 weeks and then post-OHDF for the next 3 weeks. The OHDF treatment condition parameters such as blood flow rate (QB; which does not include the dialysis fluid substitution flow rate (Qs)), total dialysate flow rate (total QD; which is the sum of the flow rate of the substitution fluid and dialysate flowing through the hemodiafilter), treatment time, and the number of treatments per week, were maintained during the study period for all patients at 280 mL/min, 500 mL/min, 4 h, and three times per week, respectively. The substitution volume was changed weekly in the following order: 24 L, 36 L, 48 L (pre-OHDF) and 6 L, 8 L and 10 L (post-OHDF). To assess the reduction ratios (RRs) and removal amounts, blood and dialysate drainage samples were collected during the second dialysis day of each week. In addition, during the third (pre-OHDF with 48 L substitution volume) and sixth (post-OHDF with 10 L substitution volume) weeks, high-sensitivity C-reactive protein (hs-CRP) and IL-6 levels were measured to assess inflammatory responses. The basis of the maximum substitution volumes of 48 L and 10 L for pre- and post-OHDF, respectively, is that the average substitution volumes of those in Japanese facilities are 39.9 L and 10.2 L respectively [19].
Reduction ratio
Pre- and post-dialysis blood samples were collected within 15 min before commencing dialysis and within 15 min after dialysis completion, respectively. The blood samples were immediately centrifuged to separate the plasma, and the plasma was then used to assay the concentrations of urea, uric acid (UA), creatinine (CRE), inorganic phosphorus (iP), beta-2-microglobulin (β2-MG), and α1-MG. The RRs of urea, UA, creatinine, and iP were calculated using the following formula:
where Cpre and Cpost are the concentrations of urea, UA, creatinine, or iP in peripheral blood in the pre- and post-dialysis phases, respectively.
The RRs of β2-MG and α1-MG were calculated using the following formula that includes hematocrit (HCT) correction:
where Cpre and Cpost are the concentrations of β2-MG and α1-MG in the peripheral plasma, and HCTpre and HCTpost are the HCT values (in %) measured at the pre- and post-dialysis sessions, respectively.
Removal amount and albumin leakage
The removal amount of β2-MG, α1-MG, and albumin leakage was calculated using the following formula:
The partial storage method was used to store the dialysate drainage [20].
To assess the selectivity of the α1-MG removal for albumin leakage, the selective removal index of α1-MG (SRIA) was calculated [20]. The SRIA was calculated as the amount of α1-MG removed divided by the amount of albumin leakage in a single session using the following formula:
Inflammatory responses
Blood samples were collected; they underwent the same treatment as for RR evaluation and were used to assay the concentrations of hs-CRP and IL-6.
Adverse events and device malfunction
All adverse events (AEs) were recorded for all treatments and interdialytic days. For example, even minor events, such as a patient’s feeling of discomfort, muscle cramps requiring minimal intervention, or changing the ultrafiltration flow rate, were recorded as AEs in the case report forms. The dialysis treatment condition parameters such as flow rates of blood and dialysate, vital signs at pre- and post-dialysis, dry weights, volumes of water removed, and transmembrane pressures (TMPs) were also recorded. All device malfunctions that occurred during any of the treatment sessions were recorded as well.
Statistical analysis
Statistical analyses for RRs, removal amounts, and albumin leakage were compared among the same dilution modes. The values for 48 L in pre-OHDF were compared in detail to those for 24 L and 36 L; similarly, the values for 10 L in post-OHDF were compared to those for 6 L and 8 L. All parameters were compared using paired t tests. Statistical significance was set at P < 0.05 (two-sided). No adjustments for multiple comparisons were performed. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., USA).
Results
Patient enrollment and population analysis
Eight patients were screened for enrollment in the study, with none deemed ineligible. The patient baseline characteristics are shown in Table 1. All patients completed the OHDF therapy using ABH-22PA for 6 weeks. None were lost to follow-up, and no deaths occurred during the study. In the following evaluations, the data for all patients were defined as the safety analysis (SAA) and full analysis set (FAS) populations. However, in the sixth week only, the removal amounts of β2-MG, α1-MG, and albumin leakage were calculated using data from seven patients because the dialysate drainage samples could not be measured in one patient. The first treatment of the study began on January 13, 2020, and the last treatment was completed on February 22, 2020.
Performance evaluation
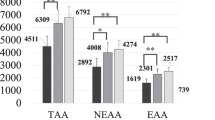
The RRs of urea, UA, creatinine, iP, β2-MG, and α1-MG in the FAS population are summarized in Table 2. The RRs of urea, UA, creatinine, iP, and β2-MG were almost the same in pre- and post-OHDF, while the RRs of α1-MG tended to be slightly higher in post-OHDF. Using the same dilution mode, the larger the substitution volume, the larger the RR of α1-MG. The removal amounts of β2-MG, α1-MG, albumin leakage, and SRIA are summarized in Table 3. The removal amount of α1-MG showed a similar trend as its RR: it was slightly higher in post-OHDF, and with the same dilution treatment, the larger the substitution volume, the larger the removal amount. The removal amounts of α1-MG and albumin leakage of pre-OHDF at 48 L were significantly higher than those of pre-OHDF at 24 L (P-values were 0.017 and 0.001, respectively), and those of post-OHDF at 10 L were also significantly higher than those of post-OHDF at 6 L (P-values of 0.034 and 0.047, respectively). However, no significant difference was observed between pre-OHDF at 48 L and 36 L or post-OHDF at 10 L and 8 L. Moreover, no significant difference was observed in the removal amounts of β2-MG between the two conditions. The SRIA was almost the same when the same dilution mode was used; however, at different dilution modes, SRIA tended to be slightly higher in pre- than in post-OHDF.
Inflammatory responses
The inflammatory responses in the FAS population are summarized in Table 4. In pre-OHDF, the values of hs-CRP and IL-6 after the treatment decreased compared with those before, whereas they increased in post-OHDF. However, no significant differences were observed between pre- and post-OHDF. The elevation in the mean values of hs-CRP in post-OHDF was observed in one patient who developed fever, as described in the AE section. The values were 1.71 mg/dL and 2.10 mg/dL before and after treatment, respectively. Moreover, the patient’s blood sampling date was the day after the onset of the fever. The median values of hs-CRP in pre- and post-OHDF were almost the same. Therefore, it can be concluded that the increase in the mean hs-CRP levels was due to the fever in this patient.
AE and device malfunction
Two AEs, suspected infectious gastroenteritis and fever, were recorded in two patients. These AEs improved within 1 day and were considered unrelated to ABH-22PA. No serious AEs or device malfunctions were recorded. Furthermore, complications due to elevated TMP, such as interruption of treatment, did not occur in either dilution mode.
Discussion
The results indicated that the removal performance varied depending on the substitution volume in pre- and post-OHDF, as in previous reports [6,7,8, 15]. In particular, the tendency was remarkable in middle molecules, which are predominantly removed by filtration rather than diffusion.
An interesting finding of this study was that the removal performance of small-molecular-size substances, such as urea, UA, CRE, and iP, was not inferior in pre-OHDF to that in post-OHDF. Theoretically, small-molecular-size substances are predominantly removed by diffusion; therefore, their removal performance should be superior in post-OHDF. The concentration of such substances is diluted in pre-OHDF, and the concentration difference between the blood side and dialysate is less in pre- than in post-OHDF. However, in this study, the reduction ratios of small-molecular-size substances were almost the same regardless of the dilution mode. This might reflect the excellent removal performance of ABH-PA through the diffusion of small-molecular-size substances using an improved three-dimensional hollow fiber structure.
Meanwhile, post-OHDF was superior to pre-OHDF in terms of removal performance of α1-MG, which is predominantly removed by filtration. The removal performance of the middle molecules increases as the substitution volume increases in the same dilution mode, and comparing the dilution modes, post-OHDF is superior to pre-OHDF. This result resonates with that of previous studies [20].
For β2-MG, unlike α1-MG, no significant difference was observed in the removal performance regardless of the dilution mode or substitution volume, as with small-molecular-size substances. Therefore, β2-MG, with a molecular weight of 11,800, was thought to be removed mainly by diffusion rather than by filtration. This is consistent with the findings of previous reports that compared the removal performance of pre- and post-OHDF [5, 20].
As β2-MG is removed mainly by diffusion, it would be insufficient to attribute the improvement in mortality resulting from OHDF to β2-MG removal only. Instead, it is necessary to also focus on the removal of substances that have a higher molecular weight than β2-MG and are mainly removed by filtration. Among these substances, clinical manifestations related to dialysis are alleviated when the reduction ratio of α1-MG exceeds 35% [5]. When attempting to increase the reduction ratio of α1-MG, albumin will inevitably leak out simultaneously. This is because the Stokes radii of free α1-MG and albumin are similar, at 28.6 Å [21] and 35.5 Å, respectively, even though their molecular weights differ, at approximately 33,000 and 66,000, respectively. α1-MG has been reported to form a complex with IgA, prothrombin, and albumin within the body [22]. However, the Stokes radii of α1-MG combined with IgA, prothrombin, and albumin remain unknown. New types of hemodiafilters that can selectively remove α1-MG with low albumin leakage are under development.
In the removal performance of the selectivity of the α1-MG removal for albumin leakage, some differences were observed between pre- and post-OHDF. SRIA, an index of the selective removal capacity of α1-MG, was slightly better in pre- than in post-OHDF. However, SRIA tends to be high when the amount of albumin leakage is low, even if the removal amount of α1-MG is minimal. In this study, the maximum average removal amount of albumin for each dilution mode was 2.46 g in pre-OHDF with 48 L substitution volume and 3.51 g in post-OHDF with 10 L substitution volume, which were too low to assess SRIA. Therefore, the variation in substitution volume in each group was not sufficient for detailed discussion, and further studies are required to elucidate the selective removal capacity of α1-MG with different dilution modes.
In this study, the levels of the inflammatory markers such as hs-CRP and IL-6, both before and after treatment, demonstrated no significant difference between pre- and post-OHDF. The inflammatory response was measured during post-OHDF at a substitution volume of 10 L, and QB was 280 mL/min; thus, the ratio of filtration flow rate (Qf) to QB was approximately 15%. This value is lower than the standard ratio in EU countries. In a study regarding EU countries, more than half of post-OHDF patients were treated with a substitution volume of ≥ 20 L, and the average QB of the patients was 342 mL/min, in which case the ratio of Qf to QB was ≥ 24% [23]. Some differences in inflammatory response may be observed under conditions of higher blood concentrations. Further studies with a high Qf to QB ratio are required to confirm this assumption.
There were no device-related AEs, device malfunctions, or elevations in TMP in either the pre- or post-OHDF modes. Therefore, both pre- and post-OHDF can be regarded as sufficiently safe for treatment under the conditions evaluated in this study.
This study has some limitations. First, no female patients were involved in the study, which means that the current findings are not representative of what occurs in both males and females. Second, the findings were associated with OHDF employing only ABH-22PA. Therefore, if other instruments are employed, the results may vary. Third, there were no data on high volumes of substitution fluid as the maximum amount of substitution fluid was based on the average in Japan. Further studies employing a larger population, the use of other instruments, and higher volumes of substitution fluid are required.
This study aimed to confirm removal performance when the same hemodiafilters were used in the same patients; thus, mortality and other clinical benefits were not evaluated. Further studies are required to evaluate the relationship between substance removal, especially of middle molecules, and mortality.
Conclusion
By using the same hemodiafilter in the same patients, the differences in removal performance between pre- and post-OHDF have become clear. Post-OHDF is superior to pre-OHDF in terms of removal performance by filtration. Meanwhile, removal performance by diffusion is almost the same, regardless of the dilution mode. Moreover, no significant difference is noted in inflammatory responses between pre- and post-OHDF.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–70.
Tattersall JE, Ward RA. Online haemodiafiltration: definition, dose quantification and safety revisited. Nephrol Dial Transplant. 2013;28:542–50.
Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–43.
Masakane I, Sakurai K. Current approaches to middle molecule removal: room for innovation. Nephrol Dial Transplant. 2018;33:iii12-21.
Sakurai K. Biomarkers for evaluation of clinical outcomes of hemodiafiltration. Blood Purif. 2013;35:64–8.
Maduell F, Moreso F, Pons M, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–97.
Grooteman MP, van den Dorpel MA, Bots ML, et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23:1087–96.
Ok E, Asci G, Toz H, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF study. Nephrol Dial Transplant. 2013;28:192–202.
Okada K, Tashiro M, Michiwaki H, et al. Effects of high albumin leakage on survival between online hemodiafiltration and super high-flux hemodialysis: the HISTORY study. Ren Replace Ther. 2022;8:52.
Masakane I, Kikuchi K, Kawanishi H. Evidence for the clinical advantages of Predilution on-line hemodiafiltration. Contrib Nephrol. 2017;189:17–23.
Gritters-van den Oever M, Grooteman MP, Bartels PC, et al. Post-dilution haemodiafiltration and low-flux haemodialysis have dissimilar effects on platelets: a side study of CONTRAST. Nephrol Dial Transplant. 2009;24:3461–8.
Sakurai K, Saito T, Yamauchi F, et al. Comparison of the effects of predilution and postdilution hemodiafiltration on neutrophils, lymphocytes and platelets. J Artif Organs. 2013;16:316–21.
Blankestijn PJ, Grooteman MP, Nube MJ, Bots ML. Clinical evidence on haemodiafiltration. Nephrol Dial Transplant. 2018;33:iii53–8.
Okada K, Michiwaki H, Tashiro M, et al. Effects of Japanese-style online hemodiafiltration on survival and cardiovascular events. Ren Replace Ther. 2021;7:70.
Kikuchi K, Hamano T, Wada A, et al. Predilution online hemodiafiltration is associated with improved survival compared with hemodialysis. Kidney Int. 2019;95:929–38.
Sakurai K, Hosoya H, Kurihara Y, et al. Evaluation of low-volume post-dilution online hemodiafiltration with Japanese high-performance hemodiafilters. J Artif Organs. 2020;23:234–9.
Santos García A, Macías Carmona N, Vega Martínez A, et al. Removal capacity of different high-flux dialyzers during postdilution online hemodiafiltration. Hemodial Int. 2019;23:50–7.
Albalate Ramón M, Martínez Miguel P, Bohorquez L, et al. Asymmetric cellulose triacetate is a safe and effective alternative for online haemodiafiltration. Nefrol (Engl Ed). 2018;38:315–20.
Masakane I, Taniguchi M, Nakai S, et al. Annual dialysis data report 2016, JSDT renal data registry. Ren Replace Ther. 2018;4:45.
Tanaka Y, Michiwaki H, Asa H, et al. Multipotentials of new asymmetric cellulose triacetate membrane for on-line hemodiafiltration both in postdilution and predilution. Ren Replace Ther. 2019;5:21.
Wester L, Johansson MU, Akerström B. Physicochemical and biochemical characterization of human alpha 1-microglobulin expressed in baculovirus-infected insect cells. Protein Expr Purif. 1997;11:95–103.
Berggård T, Thelin N, Falkenberg C, et al. Prothrombin, albumin and immunoglobulin A form covalent complexes with alpha1-microglobulin in human plasma. Eur J Biochem. 1997;245:676–83.
Locatelli F, Karaboyas A, Pisoni RL, et al. Mortality risk in patients on hemodiafiltration versus hemodialysis: a ‘real-world’ comparison from the DOPPS. Nephrol Dial Transplant. 2018;33:683–9.
Acknowledgements
We are grateful to the hemodialysis staff of the dialysis unit at Kawashima Touseki Clinic, especially Naoto Ueta, for their contribution to conducting the study. We are also grateful to Dr. Shigeaki Ohtsuki of the Japan Institute of Statistical Technology for performing the statistical analysis. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Asahi Kasei Medical Co., Ltd.; funding was provided for the research and publication of this article.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: K. Ohshima is an employee of Asahi Kasei Medical Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okada, K., Michiwaki, H., Mori, H. et al. Removal performance of pre- and post-dilution online hemodiafiltration using identical hemodiafilters in the same patients. J Artif Organs 26, 309–315 (2023). https://doi.org/10.1007/s10047-022-01379-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-022-01379-4