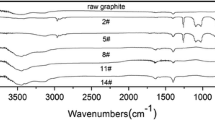
Abstract
Carbon-based structures have attracted attention due to their unique properties, combining excellent chemical, thermal mechanical, and electrical features. The use of microwave irradiation has been used to produce graphene materials and the identification of optimum synthesis parameters is crucial for the production of high-quality carbon structures. Herein, we describe a detailed temperature study for the production of expanded graphite nanosheets (GNs) via intercalation of sulfuric and nitric acids, followed by a rapid and energy-efficient process of microwave-assisted expansion and exfoliation. The results demonstrate that the proposed methodology was efficient in producing high-quality GNs with a size of approximately 1 nm. The temperature investigation revealed that heat treatment of 800 °C was the best, resulting in a high-quality material, achieving maximum expansion with the lowest weight loss related to thermal degradation. Thus, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) tests confirmed good electrochemical features of the sample produced at a temperature of 800 °C.







Similar content being viewed by others

References
Huang L, Cao Y, Diao D (2019) Surface N-doped graphene sheets induced high electrocatalytic activity for selective ascorbic acid sensing. Sens Actuators B Chem 283:556–562. https://doi.org/10.1016/j.snb.2018.12.079
Guo X, Xu Y, Cheng Y et al (2020) Amorphous cobalt phosphate porous nanosheets derived from two-dimensional cobalt phosphonate organic frameworks for high performance of oxygen evolution reaction. Appl Mater Today 18:100517. https://doi.org/10.1016/j.apmt.2019.100517
Vaughan DJ (2019) Graphite to graphene: from a mineral to an advanced technological material. Elements 15:215–216. https://doi.org/10.2138/gselements.15.3.215
Meng LY, Park SJ (2012) Preparation and characterization of reduced graphene nanosheets via pre-exfoliation of graphite flakes. Bull Korean Chem Soc 33:209–214. https://doi.org/10.5012/bkcs.2012.33.1.209
Liu C, Bai Y, Wang J et al (2021) Controllable synthesis of ultrathin layered transition metal hydroxide/zeolitic imidazolate framework-67 hybrid nanosheets for high-performance supercapacitors. J Mater Chem A 9:11201–11209. https://doi.org/10.1039/d1ta02065j
Kumar A, Sharma K, Dixit AR (2020) A review on the mechanical and thermal properties of graphene and graphene-based polymer nanocomposites: understanding of modelling and MD simulation. Mol Simul 46:136–154. https://doi.org/10.1080/08927022.2019.1680844
Poniatowska A, Trzaskowski M, Ciach T (2019) Production and properties of top-down and bottom-up graphene oxide. Colloids Surfaces A Physicochem Eng Asp 561:315–324. https://doi.org/10.1016/j.colsurfa.2018.10.049
Edwards RS, Coleman KS (2013) Graphene synthesis: relationship to applications Nanoscale 5:38–51. https://doi.org/10.1039/c2nr32629a
Trikkaliotis DG, Mitropoulos AC, Kyzas GZ (2020) Low-cost route for top-down synthesis of over- and low-oxidized graphene oxide. Colloids Surfaces A Physicochem Eng Asp 600:124928. https://doi.org/10.1016/j.colsurfa.2020.124928
Lin L, Peng H, Liu Z (2019) Synthesis challenges for graphene industry. Nat Mater 18:520–524. https://doi.org/10.1038/s41563-019-0341-4
Zhang R, Zhang B, Sun S (2015) Preparation of high-quality graphene with a large-size by sonication-free liquid-phase exfoliation of graphite with a new mechanism. RSC Adv 5:44783–44791. https://doi.org/10.1039/c5ra04480d
Lund S, Kauppila J, Sirkiä S et al (2021) Fast high-shear exfoliation of natural flake graphite with temperature control and high yield. Carbon N Y 174:123–131. https://doi.org/10.1016/j.carbon.2020.11.094
AL-Saedi SI, Haider AJ, Naje AN, Bassil N (2019) Improvement of Li-ion batteries energy storage by graphene additive. Energy Rep 6:64–71. https://doi.org/10.1016/j.egyr.2019.10.019
Chen J, Zhang Y, Zhang M et al (2016) Water-enhanced oxidation of graphite to graphene oxide with controlled species of oxygenated groups. Chem Sci 7:1874–1881. https://doi.org/10.1039/c5sc03828f
Hou B, Liu H, Qi S et al (2018) Preparation of pristine graphene in ethanol assisted by organic salts for nonenzymatic detection of hydrogen peroxide. J Colloid Interface Sci 510:103–110. https://doi.org/10.1016/j.jcis.2017.09.052
Yang S, Lohe MR, Müllen K, Feng X (2016) New-generation graphene from electrochemical approaches: production and applications. Adv Mater 28:6213–6221. https://doi.org/10.1002/adma.201505326
Paredes JI, Munuera JM (2017) Recent advances and energy-related applications of high quality/chemically doped graphenes obtained by electrochemical exfoliation methods. J Mater Chem A 5:7228–7242. https://doi.org/10.1039/c7ta01711a
Wu W, Liu M, Gu Y et al (2020) Fast chemical exfoliation of graphite to few-layer graphene with high quality and large size via a two-step microwave-assisted process. Chem Eng J 381. https://doi.org/10.1016/j.cej.2019.122592
Liu T, Zhang X, Liu M et al (2018) One-step room-temperature exfoliation of graphite to 100% few-layer graphene with high quality and large size. J Mater Chem C 6:8343–8348. https://doi.org/10.1039/c8tc02756k
Liu M, Zhang X, Wu W et al (2019) One-step chemical exfoliation of graphite to ∼100% few-layer graphene with high quality and large size at ambient temperature. Chem Eng J 355:181–185. https://doi.org/10.1016/j.cej.2018.08.146
Jiang Y, Yin X, Guan D et al (2019) Co-transport of Pb(II)and oxygen-content-controllable graphene oxide from electron-beam-irradiated graphite in saturated porous media. J Hazard Mater 375:297–304. https://doi.org/10.1016/j.jhazmat.2019.05.001
Tu J, Li H, Lan T et al (2020) Facile synthesis of TiN nanocrystals/graphene hybrid to chemically suppress the shuttle effect for lithium-sulfur batteries. J Alloys Compd 822:153751. https://doi.org/10.1016/j.jallcom.2020.153751
Liu Y, Zhang Y, Liu Y et al (2021) Super heating/cooling rate enabled by microwave shock on polymeric graphene foam for high performance lithium–sulfur batteries. Carbon N Y 173:809–816. https://doi.org/10.1016/j.carbon.2020.11.061
Jakhar R, Yap JE, Joshi R (2020) Microwave reduction of graphene oxide. Carbon N Y 170:277–293. https://doi.org/10.1016/j.carbon.2020.08.034
Xu S, Zhong G, Chen C et al (2019) Uniform, scalable, high-temperature microwave shock for nanoparticle synthesis through defect engineering. Matter 1:759–769. https://doi.org/10.1016/j.matt.2019.05.022
Liu Y, Ge Z, Li Z, Chen Y (2021) High-power instant-synthesis technology of carbon nanomaterials and nanocomposites. Nano Energy 80:105500. https://doi.org/10.1016/j.nanoen.2020.105500
Xie X, Zhou Y, Huang K (2019) Advances in microwave-assisted production of reduced graphene oxide. Front Chem 7:1–11. https://doi.org/10.3389/fchem.2019.00355
Park SH, Bak SM, Kim KH et al (2011) Solid-state microwave irradiation synthesis of high quality graphene nanosheets under hydrogen containing atmosphere. J Mater Chem 21:680–686. https://doi.org/10.1039/c0jm01007c
Wu J, Zhao J, Vaidhyanathan B et al (2020) Rapid microwave-assisted bulk production of high-quality reduced graphene oxide for lithium ion batteries. Materialia 13:100833. https://doi.org/10.1016/j.mtla.2020.100833
Lin J, Huang Y, Wang S, Chen G (2017) Microwave-assisted rapid exfoliation of graphite into graphene by using ammonium bicarbonate as the intercalation agent. Ind Eng Chem Res 56:9341–9346. https://doi.org/10.1021/acs.iecr.7b01302
Pierini GD, Foster CW, Rowley-Neale SJ et al (2018) A facile electrochemical intercalation and microwave assisted exfoliation methodology applied to screen-printed electrochemical-based sensing platforms to impart improved electroanalytical outputs. Analyst 143:3360–3365. https://doi.org/10.1039/c7an01982c
Yurddaskal M, Erol M, Celik E (2017) Carbon black and graphite filled conducting nanocomposite films for temperature sensor applications. J Mater Sci Mater Electron 28:9514–9518. https://doi.org/10.1007/s10854-017-6695-y
Liu Z, Pan F, Deng B et al (2021) Self-assembled MoS2/3D worm-like expanded graphite hybrids for high-efficiency microwave absorption. Carbon N Y 174:59–69. https://doi.org/10.1016/j.carbon.2020.12.019
Jiang F, Yu Y, Wang Y et al (2017) A novel synthesis route of graphene via microwave assisted intercalation-exfoliation of graphite. Mater Lett 200:39–42. https://doi.org/10.1016/j.matlet.2017.04.048
Knuth RD, Knuth FA, Maron GK et al (2022) Development of xanthan gum-based solid polymer electrolytes with addition of expanded graphite nanosheets. J Appl Polym Sci 139:1–12. https://doi.org/10.1002/app.52400
Garg P, Bharti SRK, Raman R (2020) Graphene oxide–silver nanocomposite SERS substrate for sensitive detection of nitro explosives. J Mater Sci Mater Electron 31:1094–1104. https://doi.org/10.1007/s10854-019-02621-1
Zhang BB, Huang MH, Dai XC et al (2019) Self-assembly of graphene-encapsulated antimony sulfide nanocomposites for photoredox catalysis: boosting charge transfer: via interface configuration modulation. New J Chem 43:13837–13849. https://doi.org/10.1039/c9nj02593f
Wang H, Wang S, Lu W et al (2018) Through-thickness thermal conductivity enhancement of graphite film/epoxy composite via short duration acidizing modification. Appl Surf Sci 442:170–177. https://doi.org/10.1016/j.apsusc.2018.02.125
Cai X, Luo Y, Liu B, Cheng HM (2018) Preparation of 2D material dispersions and their applications. Chem Soc Rev 47:6224–6266. https://doi.org/10.1039/c8cs00254a
Barzegar F, Bello A, Momodu D et al (2016) Preparation and characterization of porous carbon from expanded graphite for high energy density supercapacitor in aqueous electrolyte. J Power Sources 309:245–253. https://doi.org/10.1016/j.jpowsour.2016.01.097
Vertuccio L, De Santis F, Pantani R et al (2019) Effective de-icing skin using graphene-based flexible heater. Compos Part B Eng 162:600–610. https://doi.org/10.1016/j.compositesb.2019.01.045
Sun Z, Zhao ZK, Zhang YY et al (2021) Mechanical, tribological and thermal properties of injection molded short carbon fiber/expanded graphite/polyetherimide composites. Compos Sci Technol 201:108498. https://doi.org/10.1016/j.compscitech.2020.108498
Kumar P, Penta S, Mahapatra SP (2019) Dielectric properties of graphene oxide synthesized by modified hummers’ method from graphite powder. Integr Ferroelectr 202:41–51. https://doi.org/10.1080/10584587.2019.1674822
Ghosh S, Polaki SR, Ajikumar PK et al (2018) Aging effects on vertical graphene nanosheets and their thermal stability. Indian J Phys 92:337–342. https://doi.org/10.1007/s12648-017-1113-0
Liu Y, Wu X, Tian Y et al (2019) Largely enhanced oxidation of graphite flakes via ammonium persulfate assisted gas expansion for the preparation of graphene oxide sheets. Carbon N Y 146:618–626. https://doi.org/10.1016/j.carbon.2019.02.052
Hooch Antink W, Choi Y, Seong KD et al (2018) Recent progress in porous graphene and reduced graphene oxide-based nanomaterials for electrochemical energy storage devices. Adv Mater Interfaces 5:1–19. https://doi.org/10.1002/admi.201701212
Al-Hazmi FS, Al-Harbi GH, Beall GW et al (2015) One pot synthesis of graphene based on microwave assisted solvothermal technique. Synth Met 200:54–57. https://doi.org/10.1016/j.synthmet.2014.12.028
Shearer CJ, Slattery AD, Stapleton AJ et al (2016) Accurate thickness measurement of graphene. Nanotechnology 27:0. https://doi.org/10.1088/0957-4484/27/12/125704
Sun Y, Yang L, Xia K et al (2018) “Snowing” graphene using microwave ovens. Adv Mater 30:1–8. https://doi.org/10.1002/adma.201803189
Yang C, Chen J, Ji X et al (2019) Aqueous Li-ion battery enabled by halogen conversion–intercalation chemistry in graphite. Nature 569:245–250. https://doi.org/10.1038/s41586-019-1175-6
An Y, Fei H, Zeng G et al (2018) Commercial expanded graphite as a low–cost, long-cycling life anode for potassium–ion batteries with conventional carbonate electrolyte. J Power Sources 378:66–72. https://doi.org/10.1016/j.jpowsour.2017.12.033
da Silva JMD, Coutinho SVCR, Diniz RKM et al (2019) Evaluation of the mechanical and thermal properties of blended (HDPE/UHMWPE) nanocomposites with graphite nanosheets (GNS). Macromol Symp 383:1–8. https://doi.org/10.1002/masy.201800017
Ji Q, Wang B, Zheng Y et al (2021) Scalable fabrication of holey graphene nanosheets by electrochemical intercalation and microwave-assisted expansion of graphite. Appl Surf Sci 560:150052. https://doi.org/10.1016/j.apsusc.2021.150052
Jehad AK, Kocabas K, Yurddaskal M (2020) A comparative study for producing few-layer graphene sheets via electrochemical and microwave-assisted exfoliation from graphite powder. J Mater Sci Mater Electron 31:7022–7034. https://doi.org/10.1007/s10854-020-03268-z
Zabihi O, Ahmadi M, Li Q et al (2019) Simultaneous electrochemical-assisted exfoliation and in situ surface functionalization towards large-scale production of few-layer graphene. FlatChem 18:100132. https://doi.org/10.1016/j.flatc.2019.100132
Domga KM, Oladoyinbo F et al (2020) A simple, economical one-pot microwave assisted synthesis of nitrogen and sulfur co-doped graphene for high energy supercapacitors. Electrochim Acta 341:135999. https://doi.org/10.1016/j.electacta.2020.135999
Chen Y, Zhang A, Ding L et al (2017) A three-dimensional absorber hybrid with polar oxygen functional groups of MWNTs/graphene with enhanced microwave absorbing properties. Compos Part B Eng 108:386–392. https://doi.org/10.1016/j.compositesb.2016.10.014
Roy D, Kanojia S, Mukhopadhyay K, Eswara Prasad N (2021) Analysis of carbon-based nanomaterials using Raman spectroscopy: principles and case studies. Bull Mater Sci 44:1–9. https://doi.org/10.1007/s12034-020-02327-9
Yang K, Liu Q, Zheng Y et al (2021) Locally ordered graphitized carbon cathodes for high-capacity dual-ion batteries. Angew Chemie 133:6396–6402. https://doi.org/10.1002/ange.202016233
Xie J, Li X, Lai H et al (2019) A robust solid electrolyte interphase layer augments the ion storage capacity of bimetallic-sulfide-containing potassium-ion batteries. Angew Chemie - Int Ed 58:14740–14747. https://doi.org/10.1002/anie.201908542
Li X, Liu Z, Li J et al (2020) Insights on the mechanism of Na-ion storage in expanded graphite anode. J Energy Chem 53:56–62. https://doi.org/10.1016/j.jechem.2020.05.022
Gouda MH, Gouveia W, Afonso ML et al (2019) Poly(vinyl alcohol)-based crosslinked ternary polymer blend doped with sulfonated graphene oxide as a sustainable composite membrane for direct borohydride fuel cells. J Power Sources 432:92–101. https://doi.org/10.1016/j.jpowsour.2019.05.078
Maron GK, Alano JH, da Silveira Noremberg B et al (2020) Electrochemical supercapacitors based on 3D nanocomposites of reduced graphene oxide/carbon nanotube and ZnS. J Alloys Compd 836. https://doi.org/10.1016/j.jallcom.2020.155408
Qu R, Tang S, Qin X et al (2017) Expanded graphite supported Ni(OH)2 composites for high performance supercapacitors. J Alloys Compd 728:222–230. https://doi.org/10.1016/j.jallcom.2017.08.270
Pogacean F, Coros M, Mirel V et al (2019) Graphene-based materials produced by graphite electrochemical exfoliation in acidic solutions: application to Sunset Yellow voltammetric detection. Microchem J 147:112–120. https://doi.org/10.1016/j.microc.2019.03.007
Acknowledgements
The authors would like to thank the company (Nacional de Grafite LTDA) for the donation of Graflake, essential material for this work. We are grateful for the support of the National Council for Scientific and Technological Development (CNPq), the Financier of Studies and Projects (FINEP), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) process number 21/2551-0002237-0 and Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES)—Finance Code 001. Finally, we would like to thank the team at the CEME-Sul Laboratory at the Federal University of Rio Grande where (FE-SEM) analysis was made.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Knuth, R.D., Knuth, F.A., Maron, G.K. et al. Preparation and characterization of graphene nanosheets from graphite flakes through assisted intercalation-expansion using a microwave oven. J Solid State Electrochem (2023). https://doi.org/10.1007/s10008-023-05736-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10008-023-05736-y