Abstract
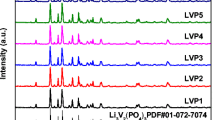
To improve capacity and electrochemical performance of the cathode of Li-ion batteries, non-stoichiometric, vanadium-excess (V-excess) Li3V2(PO4)3-LiVOPO4/C (LVP-LVOP/C) composite cathode materials are synthesized by a single-step citric acid assisted sol–gel method and sintered at temperatures (300–900 °C). X-ray diffraction and transmission electron microscope results indicate that major Li3V2(PO4)3 and minor LiVOPO4 phases coexist and X-ray photoelectron spectroscopy results also show that the valance state of vanadium is + 4 and + 3. The sample sintered at 800 °C shows the best electrochemical performance with the highest discharge capacity of 140 mAh g−1 at 0.2 C, higher than the theoretical capacity of Li3V2(PO4)3 in the voltage range 2.8–4.3 V. The composite material displays remarkably improved stability exhibiting reversible capacity of 130, 115, and 108 mAh g−1 after 300, 500, and 1000 cycles at the rate of 0.3 C, 0.5 C, and 1 C, respectively. Additionally, the composite LVP-LVOP/C shows superior rate performance at various current densities from 0.2 to 10 C. Our study reveals that the novel composite material considerably enhances electrochemical performance, electronic conductivity, Li-ion diffusion, and contribution of LiVOPO4 to capacity by accommodating extra Li-ions to enhance capacity. The results demonstrate that the study is highly promising for the development of V-excess cathodes as V-excess composite materials exhibit better performance than pure phase Li3V2(PO4)3.
Graphical abstract








Similar content being viewed by others
References
Xu X, Xiong F, Meng J et al (2020) Vanadium-based nanomaterials: a promising family for emerging metal-ion batteries. Adv Funct Mater 30:1–36. https://doi.org/10.1002/adfm.201904398
Kang B, Ceder G (2009) Battery materials for ultrafast charging and discharging. Nature 458:190–193. https://doi.org/10.1038/nature07853
Nojima A, Sano A, Kitamura H, Okada S (2019) Electrochemical characterization, structural evolution, and thermal stability of livopo4 over multiple lithium intercalations. Evergreen 6:267–274. https://doi.org/10.5109/2547346
Cai Z, Ma Y, Huang X et al (2020) High electrochemical stability Al-doped spinel LiMn2O4 cathode material for Li-ion batteries J Energy Storage 27. https://doi.org/10.1016/j.est.2019.101036
Chung S-Y, Bloking JT, Chiang Y-M (2002) Electronically conductive phospho-olivines as lithium storage electrodes. Nat Mater 1:123–128. https://doi.org/10.1038/nmat732
Li Y, Zhou Z, Ren M et al (2006) Electrochemical performance of nanocrystalline Li3V2(PO4)3/carbon composite material synthesized by a novel sol–gel method. Electrochim Acta 51:6498–6502. https://doi.org/10.1016/j.electacta.2006.04.036
Wang Y, Tang Y, Zhong B et al (2014) Facile synthesis of Li3V2(Po4) 3/C nano-flakes with high-rate performance as cathode material for Li-ion battery. J Solid State Electrochem 18:215–221. https://doi.org/10.1007/s10008-013-2249-2
Barker J, Gover RKB, Burns P, Bryan A (2005) A symmetrical lithium-ion cell based on lithium vanadium fluorophosphate, LiVPO4F. Electrochem Solid-State Lett 8:285–288. https://doi.org/10.1149/1.1897352
Kim M, Lee S, Kang B (2017) High energy density polyanion electrode material: LiVPO4O1-xFx (x ≈ 0.25) with Tavorite Structure. Chem Mater 29:4690–4699. https://doi.org/10.1021/acs.chemmater.7b00124
Ren MM, Zhou Z, Gao XP et al (2008) LiVOPO4 hollow microspheres: one-pot hydrothermal synthesis with reactants as self-sacrifice templates and lithium intercalation performances. J Phys Chem C 112:13043–13046. https://doi.org/10.1021/jp804335b
Zheng JC, Han YD, Zhang B et al (2014) Comparative investigation of microporous and nanosheet LiVOPO4as cathode materials for lithium-ion batteries. RSC Adv 4:41076–41080. https://doi.org/10.1039/c4ra06797e
He G, Bridges CA, Manthiram A (2015) Crystal chemistry of electrochemically and chemically lithiated layered αI-LiVOPO4. Chem Mater 27:6699–6707. https://doi.org/10.1021/acs.chemmater.5b02609
Shen C, Zhang B, Zheng JC et al (2015) Effect of sintering time on the synthesize of the multi-layered core-shell LiVOPO4-Li3V2(PO4)3 composite for Li-ion batteries. J Alloys Compd 622:771–776. https://doi.org/10.1016/j.jallcom.2014.10.198
El-Desoky MM, Al-Syadi AM, Al-Assiri MS et al (2016) Electrochemical performance of novel Li3V2(PO4)3 glass-ceramic nanocomposites as electrodes for energy storage devices. J Solid State Electrochem 20:2663–2671. https://doi.org/10.1007/s10008-016-3267-7
Thanh L, Huynh N, Thuy T et al (2018) Carbon-coated LiFePO 4 – carbon nanotube electrodes for high-rate Li-ion battery. 2247–2254
Quackenbush NF, Wangoh L, Scanlon DO et al (2015) Interfacial effects in ε-LixVOPO4 and evolution of the electronic structure. Chem Mater 27:8211–8219. https://doi.org/10.1021/acs.chemmater.5b02145
Kim M, Avdeev M, Kang B (2020) Multielectron-capable Li-rich polyanion material with high operating voltage: Li5V2PO4F8 for Li-ion batteries. ACS Energy Lett 5:403–410. https://doi.org/10.1021/acsenergylett.9b02451
Whittingham MS, Siu C, Ding J (2018) Can multielectron intercalation reactions be the basis of next generation batteries? Acc Chem Res 51:258–264. https://doi.org/10.1021/acs.accounts.7b00527
Tai LH, Zhao Q, Sun LQ et al (2015) A study of the electrochemical behavior at low temperature of the Li3V2(PO4)3 cathode material for Li-ion batteries. New J Chem 39:9617–9626. https://doi.org/10.1039/c5nj01895a
Huo H, Lin Z, Wu D et al (2019) Investigating the structure of an active material-carbon interface in the monoclinic Li3V2(PO4)3/C composite cathode. ACS Appl Energy Mater 2:3692–3702. https://doi.org/10.1021/acsaem.9b00410
Membreño N, Park K, Goodenough JB, Stevenson KJ (2015) Electrode/electrolyte interface of composite α-Li3V2(PO4)3 cathodes in a nonaqueous electrolyte for lithium ion batteries and the role of the carbon additive. Chem Mater 27:3332–3340. https://doi.org/10.1021/acs.chemmater.5b00447
Ahmani Ferdi C, Belaiche M, Iffer E (2021) Structural, electrochemical, electronic, and magnetic properties of monoclinic LixV2(PO4)3 for x = 3, 2, 1 using first-principles calculations. J Solid State Electrochem 25:301–313. https://doi.org/10.1007/s10008-020-04808-7
Nojima A, Sano A, Fujita S et al (2019) Evaluation of α 1-LiVOPO 4, β-LiVOPO 4, and α-LiVOPO 4 synthesized from a same precursor by hydrothermal method. J Electrochem Soc 166:A3731–A3738. https://doi.org/10.1149/2.0691915jes
Li X, Shao Z, Liu K et al (2018) Effect of F-doping on the properties of LiFePO4-x/3Fx/C cathode materials via wet mechanical agitation-assisted high-temperature ball milling method. J Solid State Electrochem 22:2837–2843. https://doi.org/10.1007/s10008-018-4001-4
Shi Y, Zhou H, Britto S et al (2019) A high-performance solid-state synthesized LiVOPO4 for lithium-ion batteries. Electrochem commun 105:106491 https://doi.org/10.1016/j.elecom.2019.106491
Shi HY, Jia Z, Wu W et al (2020) The development of vanadyl phosphate cathode materials for energy storage systems: a review. Chem - A Eur J 26:8190–8204. https://doi.org/10.1002/chem.201905706
Lin YC, Hidalgo MFV, Chu IH et al (2017) Comparison of the polymorphs of VOPO4 as multi-electron cathodes for rechargeable alkali-ion batteries. J Mater Chem A 5:17421–17431. https://doi.org/10.1039/c7ta04558a
He G, Kan WH, Manthiram A (2018) Delithiation/lithiation behaviors of three polymorphs of LiVOPO 4. Chem Commun 54:13224–13227. https://doi.org/10.1039/C8CC07446A
Sun P, Wang X, Zhu K et al (2017) Core-shell-structured Li3V2(PO4)3-LiVOPO4 nanocomposites cathode for high-rate and long-life lithium-ion batteries. RSC Adv 7:3101–3107. https://doi.org/10.1039/c6ra26790d
Sun P, Su N, Wang Y et al (2017) Synthesizing nonstoichiometric Li3-3:XV2+x(PO4)3/C as cathode materials for high-performance lithium-ion batteries by solid state reaction. RSC Adv 7:32721–32726. https://doi.org/10.1039/c7ra04842d
Sun P, Qin S, Wang X et al (2015) Off-stoichiometric Li3-3xV2+x(PO4)3/C as cathode materials for high-performance lithium-ion batteries. J Power Sources 293:922–928. https://doi.org/10.1016/j.jpowsour.2015.06.027
Zhang B, Shen C, Zheng J et al (2014) Synthesis and characterization of a multi-layer core-shell composite cathode material LiVOPO 4 -Li 3 V 2 (PO 4) 3. J Electrochem Soc 161:A748–A752. https://doi.org/10.1149/2.050405jes
Zhong S, Zhang X, Liu J, Sui Y (2020) Study on xLiVPO4F·yLi3V2(PO4)3/C composite for high-performance cathode material for lithium-ion Batteries. Front Chem 8:1–9. https://doi.org/10.3389/fchem.2020.00361
Nguyen VH, Wang WL, Gu HB (2015) Enhanced electrochemical performance of Li3V2(PO4)3 structurally converted from LiVOPO4 by graphite nanofiber addition. Ceram Int 41:5403–5413. https://doi.org/10.1016/j.ceramint.2014.12.105
Kuo HT, Bagkar NC, Liu RS et al (2008) Structural transformation of LiVOPO4 to Li3v 2(PO4)3 with enhanced capacity. J Phys Chem B 112:11250–11257. https://doi.org/10.1021/jp803210w
Liu P, Zhang H, He W et al (2019) Lithium deficiencies engineering in Li-rich layered oxide Li1.098Mn0.533Ni0.113Co0.138O2 for high-stability cathode. J Am Chem Soc 141:10876–10882. https://doi.org/10.1021/jacs.9b04974
Kuganathan N, Chroneos A (2019) Defects and dopant properties of Li3V2(PO4)3. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-018-36398-w
Huang C, Chen D, Huang Y, Guo Y (2013) Sol-gel synthesis of Li3V2(PO4) 3/C cathode materials with high electrical conductivity. Electrochim Acta 100:1–9. https://doi.org/10.1016/j.electacta.2013.03.073
Ni Q, Bai Y, Yang Z et al (2017) Wet-chemical coordination synthesized Li3V2(PO4)3/C for Li-ion battery cathodes. J Alloys Compd 729:49–56. https://doi.org/10.1016/j.jallcom.2017.09.106
Zhou H, Shi Y, Xin F et al (2017) ϵ- and β-LiVOPO4: phase transformation and electrochemistry. ACS Appl Mater Interfaces 9:28537–28541. https://doi.org/10.1021/acsami.7b07895
Funding
This research is financially supported by Anhui Natural Science Foundation (No. 1908085ME151, KJ2020A0263), China Postdoctoral Science Foundation (No. 2020M673404), Anhui province high-end talent grant (DT18100044), the national level foreign expert introduction plan project (G20190219004), and by National Natural Science Foundation of China through Grant No. 51802322.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahsan, Z., Cai, Z., Wang, S. et al. Structural and electrochemical characterization of vanadium-excess Li3V2(PO4)3-LiVOPO4/C composite cathode material synthesized by sol–gel method. J Solid State Electrochem 25, 2127–2137 (2021). https://doi.org/10.1007/s10008-021-04986-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-021-04986-y