Abstract
In the present work, the cellulose-based materials were manufactured and used as components of electrochemical double layer capacitors (EDLCs). The preparation method of cellulose membranes as well as composite electrodes containing cellulose as a binder was presented. These materials were prepared using for the first time ionic liquid/dimethyl sulfoxide (IL/DMSO) mixture solvent. Obtained components displayed a uniform structure, thermal stability, and good electrochemical properties. The electrochemical performances of these materials were studied in 2-electrode EDLC cells by common electrochemical techniques as cyclic voltammetry (CV), galvanostatic charge/discharge (GCD), and electrochemical impedance spectroscopy (EIS). The composite electrodes were investigated in three types of electrolytes: aqueous, organic, and ionic liquids. The cellulose membranes were, however, soaked in an aqueous electrolyte and tested as hydrogel polymer electrolytes. All investigated materials show high efficiency in terms of specific capacity. The studied cellulose-based capacitors exhibited specific capacitance values in the range of 20–22 F g−1, depending on the type of applied electrolyte.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electric double layer capacitors (EDLCs), also called ultra- or supercapacitors are energy storage devices which feature high power density, reasonable energy density, excellent rate capability, low maintenance cost, and long life-cycle stability [1,2,3]. The energy storage mechanism of supercapacitors is based on reversible ion electrosorption at the electrode-electrolyte interface and/or fast and reversible redox reactions on the electroactive surface [4,5,6]. The EDLCs are widely applied in stationary power back-up systems, hybrid or electric vehicles and also could be considered as a supporting component to batteries in a variety of portable storage devices [7,8,9,10].
A typical EDLC consists of two electrodes based on high surface materials (e. g. activated carbon), separated by a porous, electrically inert, and ion-permeable separator soaked in an electrolyte. An EDLC electrode usually is composed of an active material, a conductive agent, and a binder [11]. During the last years, most of the EDLC research was focused on the development of carbonaceous materials and the investigation of their performance as an active electrode material [12,13,14]. Activated carbon is the most widely used active material for the EDLC electrodes due to its high surface area and relatively low cost [15]. Carbon materials for electrochemical energy storage devices are mainly derived from biomass [16,17,18,19]. At the same time, new types of electrolytes were studied, with the aim to increase the operative voltage of EDLC devices as a consequence of high conductivity and excellent electrochemical stability of these electrolytes [20, 21]. Liquid electrolytes are widely used in supercapacitors and are categorized into aqueous, organic, and ionic liquid electrolytes. However, their application in portable energy storage devices involves the risk of leakage. One of the methods to reduce electrolyte leakage from the electrochemical storage device is the formation of a gel polymer electrolyte (GPE) by placing a liquid electrolyte into a polymeric matrix. Gel electrolytes exhibit liquid-like ionic conductivity mechanism and can provide shape flexibility of a solid system. It functions as both a separator and an ionic conductor between electrodes [22,23,24,25,26].
Less attention was paid to the influence of a binder on performance and overall safety of the EDLC devices. The role of the binder is often underrated because it is an electrochemically inactive material whose only function is binding the electrode components together. It also represents a minor part of the whole mass of the EDLC electrode (< 10 wt%). However, binders play a crucial role in the electrode manufacturing. A good binder should provide integrity between the components in the electric network of the composite electrode, allow high adhesion of the active material of the electrode to the current collector, and must be electrochemically and chemically stable. Also, the overall safety and the environmental friendliness of these materials are very important [27,28,29,30]. The commonly used fluorinated thermoplastic binders such as polytetrafluoroethylene (PTFE) and poly(vinylidene fluoride) (PVdF) could be the source of possible pollution [31], and the EDLC electrodes based on these binders should be properly disposed of at the end of the device’s lifespan. Additionally, the binder determines the choice of solvent for the electrode preparation. Good homogeneity of the electrode slurry is crucial in this process. While PTFE is usually used in the water suspension, PVdF requires the use of expensive and toxic organic solvents like N-methyl-2-pyrrolidone (NMP). For this reason, the increasing interest is being shown in developing fluorine-free binders.
As an alternative for fluorine-based binders for the EDLC electrodes, several environmentally benign and economically viable materials were proposed, like water-soluble polymers: polyacrylic acid (PAA), polyvinyl alcohol (PVA), or cellulose-derivatives as sodium-carboxymethyl cellulose (CMC) [31,32,33,34]. For most of the abovementioned materials, the use of hazardous organic solvents is replaced by aqueous slurries in the electrode preparation process. However, because of the water-soluble property, these electrodes cannot be used in devices based on aqueous electrolytes.
Low production costs and utilization of environmentally benign substrates for manufacturing of energy storage devices are the key parameters. Cellulose is an abundant and cheap biopolymer which has the attractive properties such as biocompatibility, biodegradability, or thermal and chemical stability. It is, thus, an excellent material for the production of cheap and eco-friendly devices. Over the last years, the cellulose and its derivatives have been intensively employed as binders of carbon-based electrodes of lithium-ion batteries as well as separators or electrolyte membranes of these devices [35,36,37,38]. Recently, the natural cellulose was also introduced as a green binder of the EDLC electrode, compatible with all kinds of electrolytes employable in EDLCs (aqueous, organic and ionic liquid electrolytes) [39,40,41]. It is worth mentioning that EDLC electrode performance depends on the type of cellulose used in the capacitors. According to Wang et al. [42], cellulose microfibers (CMFs) are used to obtain electrodes with relatively low active mass loadings. In contrast, nanocellulose fiber (NCF)- and cellulose nanocrystal (CNC)-based electrodes indicate relatively high active mass loadings.
The lack of solubility in common solvents limits the easy replacement of traditional binders by the natural cellulose. An alternative electrode preparation method, with respect to the conventional processes, is therefore needed. This issue could be overcome by the procedure which was first proposed for the lithium-ion battery electrodes [43]. In accordance with the proposed method, an acetate-based ionic liquid is used as a cellulose solvent. The obtained cellulosic solution and mixture of active and conductive additives are used for slurry preparation. Then, the slurry is cast on a collector material, and finally, the composite film is formed due to contact of deionized water. The ionic liquid is exchanged in the water and then recovered.
In this paper, we present the preparation of the EDLC electrodes based on activated carbon as an electro-active material and cellulose as a fluorine-free binder. The method of electrode preparation was similar to the method described previously [44] with a slight modification. For the first time, instead of a neat ionic liquid, the IL/DMSO mixture solvent was used to obtain cellulosic solution prior to the slurry preparation. Neat ILs show defects in the context of cellulose dissolution, like high viscosity and high processing temperature. Our method allows overcoming these disadvantages by the use of IL/DMSO mixture solvent, which has a lower viscosity and a higher cellulose dissolving rate at a relatively low temperature. Additionally, it makes it possible to reduce the amount of IL and energy consumption. As a component in IL/DMSO mixture solvent, we used newly synthesized ionic liquid, N,N′-dimethyl-N-ethylpiperazinium acetate ([EDMPpz][Ac]) instead of commonly used 1-ethyl-3-methylimidazolium acetate ([EMIM][Ac]). Afterwards, the electrochemical characteristics of the prepared composite electrodes were investigated in aqueous, organic, and ionic liquid-based electrolytes.
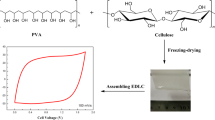
We also report the preparation of cellulose membranes, which have the potential to be applied in the EDLC devices as a hydrogel polymer electrolyte. In the case of lithium-ion batteries, the use of cellulose as the gel polymer electrolyte has already been reported [45]. Our membranes were obtained by the casting techniques described in detail elsewhere [46]. Microcrystalline cellulose (MCC) was dissolved in the same IL/DMSO mixed solution as for the preparation of composite electrodes. The obtained cellulose membranes were soaked in lithium acetate aqueous solution and tested as hydrogel electrolyte in the EDLC devices. Further, structure and thermal stability of the composite materials and the cellulose membranes were characterized by using various techniques.
Experimental section
Chemicals
N,N′-Dimethylpiperazine and bromoethane were purchased from Sigma-Aldrich and distilled before use. Acetic acid (P.O.Ch, Poland) was used as received. Dimethyl sulfoxide (P.O.Ch. Poland), acetonitrile (Sigma-Aldrich) chromatographic grade, hexane (P.O.Ch. Poland), and tetrahydrofuran (Sigma-Aldrich) were used as received. Anion exchange resin (Dowex® Monosphere 550 A) was purchased from Sigma-Aldrich and washed with distilled water before use. NaOH (P.O.Ch, Poland) was used as received. Microcrystalline cellulose powder (MCC, 20 μm, ∼ 250 degree of polymerization) was obtained from Sigma-Aldrich and used after being dried overnight at 80 °C. We also used poly(vinylidene fluoride) (PVdF) powder (Sigma-Aldrich), poly(tetrafluoroethylene) (PTFE) 60 wt% dispersion in water (Sigma-Aldrich), N-methyl-pyrrolidone (NMP) (Sigma-Aldrich), and ethanol (Chempur, Poland) for the preparation of fluorinated thermoplastic binders. As electrolytes, we employed 2 M LiAc (purity ≥ 99%, Sigma-Aldrich) aqueous solution, 1 M Et4NBF4 (purity ≥ 99%, Sigma-Aldrich) organic solution, and neat [EMIM][BF4] (high purity, Merck). Propylene carbonate (Sigma-Aldrich) was used as an organic solvent. [MPPyrr][TFSI] was synthesized in our laboratory as described in reference [14].
Ionic liquid synthesis
The cellulose required further processing before use. To prepare a cellulosic solution, IL/DMSO mixed solvent was used. N,N′-Dimethyl-N-ethylpiperazinium acetate ([DMEPpz][Ac]) ionic liquid was employed in that mixture. [DMEPpz][Ac] was prepared in a two-step reaction similarly to the method described elsewhere [47]. The first step was obtaining N,N′-dimethyl-N-ethylpiperazinium bromide ([DMEPpz][Br]). Then, an aqueous solution of the intermediate product was passing through an anion exchange resin and finally neutralized with acetic acid by acid-base reaction. After water vaporization on a rotating vacuum drier, ionic liquid was dried under vacuum at 60 °C for 24 h.
[DMEPpz][Ac] was characterized by 1H and 13C NMR Spectroscopy, FT IR spectroscopy, and electrospray-ionization mass spectrometry. 1H and 13C NMR spectra were recorded on a Varian VNMR-S 400 MHz spectrometer, in d6-DMSO. Tetramethylsilane (TMS) was used as an internal standard. The FTIR measurement was performed with a Bruker Vertex 70 FTIR spectrometer equipped with an ATR accessory. Electrospray mass spectroscopy was carried out on an AB Sciex API 4000 LC/MS/MS mass spectrometer.
1H NMR (400 MHz, DMSO-d6, δ/ppm relative to TMS): 1.25 (t, J = 7.22 Hz, 3 H) 1.57 (s, 3 H) 2.27 (s, 3 H) 2.51–2.82 (m, 4 H) 3.11 (s, 3 H) 3.32–3.48 (m, 2 H) 3.48–3.56 (m, 2 H) 3.59 (q, J = 7.28 Hz, 2 H). 13C NMR (400 MHz, DMSO-d6, δ/ppm relative to TMS): 7.05, 26.11, 44.47, 47.58, 58.36, 172.82. FT IR (ATR, neat IL, wavenumber cm−1): 3305 (br m), 2987 (vw), 2954 (vw), 2824 (vw), 1657 (w), 1563 (s), 1472 (m), 1394 (s), 1338 (vw), 1294 (w), 1253 (vw), 1209 (vw), 1192 (vw), 1147 (m), 1123 (w), 1078 (w), 1046 (w), 991 (m), 965 (w), 909 (w), 875 (m), 813 (vw), 773 (vw), 645 (vw), 466 (br s). Electrospray MS (molecular ions): ES+: m/z 143 ([DMEPpz]+). ES−: m/z 59 (CH3COO−).
Preparation of cellulose membranes
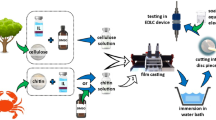
The cellulose membranes were prepared by a solution casting method. The 5 wt% cellulosic solution was obtained by dissolving a certain amount of microcrystalline cellulose in IL/DMSO mixed solvent (the weight ratio of IL in the mixed solvent system (WIL:DMSO) was equalled to 0.25). The mixture was stirred in a thermostatic block of thermo-shaker (Thermo Shaker TS100, NOVAZYM POLAND) at 50 °C for 30 min. The transparent cellulose solution was then hot-cast on a glass plate by casting knife (with the casting thickness 250 μm) and immediately immersed in deionized water in order to remove the solvent and coagulate cellulose film. Finally, the regenerated cellulose film was removed from the water, put between two Teflon sheets, and dried at 80 °C for 24 h. To form electrolyte membranes, 12-mm diameter disc-shaped pieces were cut out from cellulose film and immersed in 2 M lithium acetate (LiAc) aqueous solution. The preparation procedure is shown in Scheme 1.
Preparation of composite electrodes
Composite electrodes were prepared using activated carbon AC (powder; Norit DLC Supra 30; specific surface area 2066 m2 g−1) as an active material, acetylene black AB (Alfa Aesar; 50% compressed) as a conductive agent, and microcrystalline cellulose MCC (Sigma-Aldrich) as a binder. The preparation procedure is illustrated in Scheme 1. Prior to the slurry preparation, activated carbon and acetylene black were dry-mixed for 30 min in a laboratory shaker (IKA, Germany). The binder solution was obtained similarly to the procedure which was proposed for the cellulose membrane preparation, with a slight modification in the solvent composition. In order to reduce the IL usage, a 1 wt% cellulose solution was prepared in the IL/DMSO mixed solvent with WIL:DMSO = 0.1. The mixture of activated carbon and acetylene black was added to the binder solution and homogenized for 12 h. The obtained black slurry was cast on a conductive carbon coated aluminum foil by using a casting knife with the casting thickness of 250 μm. Subsequently, the electrode material was left overnight in a laboratory dryer at 50 °C to remove DMSO by evaporation. The coated electrode was then immersed in deionized water in order to dissolve IL in the aqueous phase. After 30 min, the electrode material was removed from the water and dried at ambient temperature for 2 h. After that, it was transferred into a laboratory dryer and left for overnight drying at 120 °C. The composition of the dry electrodes was 85 wt% AC, 5 wt% AB, and 10 wt% MCC. Electrodes were cut out into 10-mm diameter discs for further tests.
For the purposes of comparison, electrodes with the same composition, but including commonly used fluorinated thermoplastic binders such as poly(vinylidene fluoride) (PVdF) or polytetrafluoroethylene (PTFE), were prepared according to the methods described in literature [32]. All composite electrodes possessed similar values of loading (ca. 3 mg AC per single electrode).
Additionally, an activated carbon cloth (Kynol® Europa GmbH, ACC-507-20; specific surface area 2000 m2 g−1) was used as an electrode material for capacitors based on aqueous electrolytes. The activated carbon cloth was cut into 10-mm discs and saturated with an electrolyte before use. In case of these electrodes, platinum discs were used as current collectors.
Morphology of cellulose films and cellulose-based electrode materials
The morphology of cellulose-based material samples was investigated by scanning electron microscopy (SEM). SEM images were taken using a Hitachi S-3400N scanning electron microscope.
Thermal characterization
Thermogravimetric measurements were carried out using a TGA 4000 Thermogravimetric Analyzer (PerkinElmer, USA). The experiment was conducted using 3.5–4.5 mg samples of cellulose film or composite material (AC/AB/MCC). The operating conditions were kept constant for all samples. The temperature was scanned from 25 to 600 °C at a fixed heated rate of 10 °C min−1, under a nitrogen purge at a flow rate of 20 ml min−1.
Swelling of cellulose membranes in aqueous electrolyte
The hydrophilic character of obtained cellulose membranes was observed. These materials could swallow aqueous solutions, but they were resistant to common organic solvents and ionic liquids. To form electrolyte membranes, the cellulose film was immersed in 2 M LiAc aqueous solution. The degree of swelling and the swelling ratio in the direction of length and thickness of cellulose-based membranes were measured by the following procedure. The 12-mm diameter disc-shaped pieces of a dry cellulose film were weighed and their thickness was measured. Then, these discs were immersed in 2 M LiAc aqueous solution overnight. Afterwards, the weight, diameter, and thickness of obtained cellulose-based membranes were determined (the excess of electrolyte from the membrane surface was removed by a piece of tissue paper) and used to calculate the degree of swelling and the swelling ratios.
Conductivity test of cellulose membranes
The ionic conductivity of cellulose membranes was measured by the electrochemical impedance spectroscopy (EIS) method in the conductivity cell, with two parallel platinum electrodes at ambient temperature. Disc-shaped cellulose membranes soaked in 2 M LiAc were placed between two platinum electrodes. The working electrode was a 1.5-mm diameter platinum wire, and the counter was a 20-mm diameter platinum disc. To compare, the ionic conductivity of 2 M LiAc aqueous electrolyte was measured by the EIS method in the conductivity cell, with two parallel platinum electrodes at 25 °C.
Electrochemical performance
Electrochemical performances were carried out in Swagelok®-type 2-electrode cells. The symmetric EDLC cells with different composition were assembled. The cellulose membranes were tested in EDLC cells using an activated carbon cloth (Kynol® Europa GmbH, ACC-507-20; specific surface area 2000 m2 g−1) or a composite material (AC/AB/MCC). In addition, composite electrode materials were tested in EDLC cells using a commercial separator (GF/A, Whatman) soaked in different kinds of electrolytes: 2 M lithium acetate (LiAc) aqueous solution, 1 M tetraethylammonium tetrafluoroborate (Et4NBF4) organic solution (PC (propylene carbonate)), and neat ionic liquid—N-methyl-N-propylpyrrolidinium bis(trifluoromethanesulfonyl)imide ([MPPyrr][TFSI]) or 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF4]).
The electrochemical performances of EDLCs were characterized by cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charge/discharge (GCD) techniques. CV and EIS measurements were carried out using a potentiostat/galvanostat Interface1000 device (Gamry Instruments, USA), and galvanostatic measurements were taken using an Atlas 0461 MBI multichannel electrochemical system (Atlas-Sollich, Poland). Potentiodynamic measurements were taken at sweep rates from 5 to 100 mV s−1, and galvanostatic cycling was carried out at constant current densities in the range of 0.5–1.0 A g−1. All these tests were performed in the voltage range from 0 to 3.0 V (maximum voltage was dependent on the type of applied electrolyte) at ambient conditions. The specific capacitances of investigated capacitors were calculated according to the equation
in the case of potentiodynamic measurements and from the equation
in the case of galvanostatic measurements, where I is the current, dV/dt is the sweep rate of the potential, ∆V/∆t is the slope of potential change, and mE is the mass of individual electrode. The uncertainty in specific capacitance calculation was ± 1 F g−1. EIS tests were carried out applying a potential amplitude of 10 mV and frequency range from 0.01 Hz to 100 kHz at ambient conditions.
Results and discussion
Morphology, wettability, conductivity, thermal stability
Figure 1 shows the SEM images of the cellulose film and the composite electrode using cellulose as a binder. The cellulose film (in the dry state) has highly smooth and compact surface without flaws (Fig. 1a). The magnified SEM images suggest that cellulose material does not contain pores above 1 μm. Additionally, BET surface of the cellulosic film was determined, but the specific surface was below 0.1 m2 g−1, what is in agreement with literature data 0.75 m2 g−1 [48]. Furthermore, the cross section of the cellulose film indicates a dense architecture of this material (Fig. 1c). The image of the composite electrode (Fig. 1b, d) shows well distributed conductive additives which surround active material particles placed in the cellulose matrix. No aggregation of activated carbon particles is observed. The active and conductive particles form a fine network system in the cellulose matrix, therefore establishing a good quality electrode material for EDLCs.
The disc-shaped cellulose film samples were filled with 2 M lithium acetate (LiAc) aqueous solution to form cellulose membranes. As was observed, a significant amount of electrolyte was absorbed during the swelling process, resulting in an increase of membrane weight and thickness. Furthermore, a slight increase in one direction (the diameter) of the cellulose membrane was caused by the absorption of the electrolyte. High electrolyte uptake could help in achieving high ionic conductivity of the cellulose membrane, which is an important parameter of gel polymer electrolytes. However, the significant growth in the diameter of a gel polymer electrolyte, at the cost of thickness is unwanted due to the possibility of a short circuit in an energy storage device. The degree of the swelling and the swelling ratios in diameter and thickness of the cellulose membranes are shown in Table 1.
In Fig. 2, the TGA (thermogravimetric analysis) curves of the dry cellulose film and the composite electrode are presented. As could be seen, the initial part of TGA curves presents the weight loss through the presence of moisture in the sample. The major weight loss for both materials is observed above 300 °C, where degradation of both samples appears. The cellulose film in a given measurement range lost most of its weight, while the weight loss of the composite material was only several percent (approximately 15 wt%) in given conditions. It indicates that the presence of a cellulose binder in the composite material is the main responsible for the weight loss of tested samples. Thermal stability of investigated electrodes is limited by the temperature of cellulose decomposition.
Table 2 presents the specific conductivities of the 2 M LiAc aqueous solution and the hydrogel cellulose membrane soaked in this aqueous electrolyte. As can be seen, the obtained hydrogel electrolyte has a similar value of the specific conductivity as the aqueous electrolyte (electrolyte, which was used to receive that hydrogel electrolyte). The specific conductivity of 2 M LiAc aqueous solution and tested hydrogel polymer electrolyte reaches 38.2 ± 0.9 and 39.8 ± 2.8 mS cm−1, respectively. It indicates that cellulose membrane exhibits liquid-like ionic conductivity and that is a promising component for energy storage devices.
Electrochemical performance
Figure 3 presents an electrochemical performance of symmetric EDLCs containing activated carbon cloth as an electrode material and two different kinds of electrolytes: the liquid electrolyte (Whatman glass fiber separator soaked in 2 M LiAc aqueous solution) or the gel electrolyte (cellulose membrane soaked in 2 M LiAc aqueous solution). As can be seen, the EDLCs based on gel electrolytes show comparable electrochemical characteristics as the devices employing liquid electrolytes. The cyclic voltammograms (Fig. 3a, b) for both EDLCs systems show a box-type shape with an excellent charge propagation. The charge propagation decreases with the increasing scan rate (from 5 to 100 mV s−1), and a deviation from a rectangular shape of CV curves is observed for both devices, which is a typical capacitor behavior. The capacitance retention (Fig. 3c) values calculated from the cyclic voltammograms are similar for both EDLC systems. The capacitance of those systems only slightly decreases with the increasing scan rate, and it displays more than 95% of their initial capacitance at 100 mV s−1. Also, the Nyquist plot proved that efficiency of all those systems is similar. The impedance spectra of EDLCs based on the liquid electrolyte and the gel electrolyte look almost identical. Each of these impedance spectra is shifted towards the lower impedance values, and it is a straight line in the low-frequency region indicating good capacitive behaviour and low internal resistance of these devices. The cycling stability of the capacitors containing the cellulose membrane was measured by the galvanostatic charge/discharge method (GCD). High stability of the investigated capacitors over 10,000 galvanostatic charge/discharge cycles was noticed (Fig. 3d). The typical triangular shape of the charge/discharge curves with a small potential drop for that system is observed (Fig. 3d inset). The shape of the GCD curves is almost identical over 10,000 cycles, which also indicates good cyclic stability of EDLC based on the cellulose membrane.
Comparison of electrochemical performance of EDLCs based on activated carbon cloth as an electrode material. Cyclic voltammograms of EDLCs containing 2 M LiAc aqueous electrolyte (a) or hydrogel cellulose electrolyte (b). Capacitance retention of EDLCs (c) calculated from CVs (a, b) at scan rates from 5 to 100 mV s−1 and (inset) impedance curves (with a potential amplitude of 10 mV) obtained for these capacitors. Cycling stability (d) over 10,000 cycles at 0.5 A g−1 and (inset) galvanostatic charge/discharge curves of EDLC based on hydrogel cellulose electrolyte at 10th and 10,000th cycles
Furthermore, the electrochemical performance of symmetric EDLCs containing the composite electrode material (activated carbon/acetylene black/cellulose) and the liquid electrolyte (glass fiber separator (Whatman) soaked in 2 M LiAc aqueous solution) or the gel electrolyte (cellulose membrane soaked in 2 M LiAc aqueous solution) was studied by CV, GCD, and EIS analysis (Fig. 4). Similarly, as for the EDLCs containing activated carbon cloth as an electrode material, the cyclic voltammograms of the capacitors employing composite electrodes show a rectangular shape indicating good charge propagation, but the shape deviation of CV curves with the increasing scan rate is more noticeable compared to former systems (Fig. 4a, b). The reason could be the higher internal resistance of the composite electrode-based systems which can be also observed in Nyquist plot of those systems (Fig. 4c). The low-frequency regions of impedance spectra are shifted towards higher impedance values, which means that the sum of initial resistances (EDR) is higher. The CV profiles and the impedance spectra of EDLCs containing composite electrodes and the liquid electrolyte or the gel electrolyte are similar, and the capacitance retention calculated from CVs for those systems only slightly decreases with the increasing scan rate. Also, the cycling stability of the composite electrode material-based capacitors was measured (Fig. 4d). The typical triangular shape profiles of charge/discharge curves with a small, but higher than in the case of carbon cloth-based EDLC potential drops were recorded by the GCD technique (Fig. 4d inset). Upon 10,000 galvanostatic charge/discharge cycles, the electrochemical efficiency of both of those devices provides sufficient capacitance retention and barely changed GCD profiles. It indicates that those cellulose-based systems are able to undergo thousands of charge and discharge cycles with good cycle stability. Also, it is worth mentioning that the cycle stability over thousands of GCD cycles is comparable between composite electrode-based systems employing commercial glass fiber separator and cellulose membrane. Table 3 summarizes the values of specific capacitance and the values of EDR for the liquid electrolyte and the gel electrolyte-based EDLCs containing the carbon cloth material or the composite AC/AB/MCC as an electrode.
Comparison of electrochemical performance of EDLCs containing cellulose-based composite electrodes. Cyclic voltammograms of EDLCs employing 2 M LiAc aqueous electrolyte (a) or hydrogel cellulose electrolyte (b). Capacitance retention of EDLCs (c) calculated from CVs (a, b) at scan rates from 5 to 100 mV s−1 and (inset) impedance curves (with a potential amplitude of 10 mV) obtained for these capacitors. Cycling stability (d) over 10,000 cycles at 0.5 A g−1 current density and (inset) galvanostatic charge/discharge curves of EDLCs containing Whatman separator (S_W) and cellulose membrane (S_MCC) at 10th and 10,000th cycles
As shown above, the cellulose can be used as one or both of the following components of EDCL—as the binder in composite electrodes and as the biopolymer membrane soaked in an aqueous electrolyte. The cellulose film is resistant to common organic electrolytes and ionic liquids. Therefore, it cannot be used as a membrane with these types of electrolytes. However, the composite electrodes containing cellulose as the binder might be applied to EDLC devices employing all common types of electrolytes. We investigated the electrochemical performance of EDLCs based on the composite electrodes and four different electrolytes: 2 M lithium acetate (LiAc) aqueous solution, 1 M tetraethylammonium tetrafluoroborate (Et4NBF4) organic solution (PC (propylene carbonate)), and neat ionic liquids—N-methyl-N-propylpyrrolidinium bis(trifluoromethanesulfonyl)imide ([MPPyrr][TFSI]) or 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF4]). The electrodes of all tested devices were separated by glass-fiber separator soaked in the corresponding electrolyte.
Figure 5a compares the CV profiles of the EDLCs containing composite electrodes in the four considered electrolytes at a scan rate of 10 mV s−1. Electrochemical performances in 2 M LiAc aqueous solution, 1 M Et4NBF4 organic solution, and two neat ionic liquids—[MPPyrr][TFSI] and [EMIM][BF4] were studied in the potential range from 0 to 0.8, 2.4, and 3.0 V, respectively. The properties of the used electrolytes had an influence on the shape of CV curves. The difference is most notable between systems which used ionic liquids as electrolytes and the rest of tested capacitors. The CV profiles recorded for aqueous and organic-based EDLCs have a similar, rectangular shape, and the capacitance retention values (Fig. 5b) calculated from cyclic voltammograms for both of those systems are high in the whole scan range (in scan rate from 5 to 100 mV s−1). As the [MPPyrr][TFSI] has the high viscosity and the lowest conductivity of all of the investigated electrolytes, the deviation from rectangular shape of the CV of EDLCs based on this electrolyte is observed and the capacitance retention of these EDLCs was lower than in other tested systems. Because [EMIM][BF4] electrolyte has a moderate value of conductivity, the capacitance of this EDLC system decreases with the increasing scan rate more extensively than in the case of investigated aqueous or organic electrolytes, but remarkably less than in the case of [MPPyrr][TFSI]-based system.
Figure 6 shows the Nyquist plots for all tested EDLCs containing the composite electrodes in different electrolytes. As can be seen, the impedance spectrum for each studied capacitor has indicated a typical capacitive behaviour with a straight line in the low-frequency region. The aqueous electrolyte-based capacitors have shown the best electrochemical performance due to the low internal resistance of those devices. The impedance spectrum of organic electrolyte based capacitor is slightly shifted towards higher impedance values, but the highest internal resistance occurred in IL-based EDLCs, due to low conductivity of these electrolytes. Small semicircles observed on the impedance spectra at the higher frequency regions can be associated with cavity shape size of the carbon structures [49] or ion diffusion limitations [50, 51]. In the case of ILs tested in EDLC application, the explanations of depressed semicircles on EIS spectra are based on relatively high viscosity of these electrolytes [52,53,54,55,56,57]. The values of specific capacitance and the values of EDR for all composite electrode based-systems determined from EIS method are summarized in Table 4.
AC impedance curves (with a potential amplitude of 10 mV) for capacitors employing composite-based electrodes and 2 M LiAc (H2O), 1 M Et4NBF4 (PC), [EMIM][BF4] (neat), or [MPPyrr][TFSI] (neat) electrolyte. In the case of LiAc, Whatman separator (LiAc (H2O) S_W) and cellulose membrane (LiAc (H2O) S_MCC) were used
Further, the life cycle of EDLCs containing the composite electrodes in various types of electrolytes was investigated by galvanostatic charge/discharge technique. The results of presented tests were performed at 0.5 A g−1 current density and for all systems, 10,000 cycles were carried out. As shown in Fig. 7, the profiles of charge/discharge curves for all EDLCs had typical triangular shape. The highest potential drops were observed for [MPPyrr][TFSI] electrolyte-based EDLC due to the relatively low conductivity of this ionic liquid. After 10,000 cycles, all investigated systems showed performance comparable to its initial one. The shape of charge/discharge profiles for those devices has only barely changed due to slight rise of internal resistance after 10,000 cycles. It suggests that obtained electrode material with cellulose binder appears to be a promising component of electrochemical capacitors. Table 5 summarizes the values of specific capacitance for the composite electrode-based EDLCs calculated from the GCD measurements.
Galvanostatic charge/discharge (GCD) curves of EDLCs based on composite electrodes and 2 M LiAc (H2O) (black line), 1 M Et4NBF4 (PC) (red line), [EMIM][BF4] (neat) (blue line), or [MPPyrr][TFSI] (neat) (brown line) electrolyte over 10th (straight line) and 10,000th (dotted line) cycle at 0.5 A g−1 current density
Figure 8 presents SEM analysis of AC/AB/MCC composite electrodes applied for supercapacitors employing various types of electrolytes. As it is displayed by SEM images (Fig. 8b–e), the surface morphology is comparable for all investigated materials. After thousands of GCD cycles, they appear in mint conditions. Furthermore, the surface of these EDLC electrodes after galvanostatic cycling looks nearly unchanged with regard to the surface of fresh electrode (Fig. 8a).
Table 6 shows a comparison of the specific capacitance values obtained for EDLCs employing different binders of the carbon-based electrode materials. The electrochemical performance of cellulose-based EDLCs investigated in this study is very similar to that of capacitors based on fluorinated electrode binders. We also demonstrate that devices employing all the above mentioned binders achieve excellent cycle stability and after thousands of charge and discharge cycles display more than 95% of their initial value. Our results clearly prove the potential of the use of cellulose as a replacement for conventional fluorinated binders. Additionally, the performance of capacitors employing cellulose binders, which has been reported in different research papers, is comparable to that of our devices. It indicates that apart from environmental benefits, the proposed electrode preparation method allows obtaining materials with good electrochemical properties.
Conclusions
Cellulose-based materials were prepared using ionic liquids/dimethyl sulfoxide mixture solvents and applied in electrochemical double layer capacitors. Two types of EDLC components were obtained: cellulose membranes and composite electrodes containing cellulose as the binder. These materials were found to possess uniform structure, thermal stability, and good electrochemical properties. Electrochemical characteristics of devices based on those materials and containing various types of electrolytes show high efficiency in terms of specific capacitance and cyclability. The results of these studies suggest that cellulose-based materials appear to be a promising component of electrochemical capacitors.
References
Conway BE (1999) Electrochemical supercapacitors. Kluwer Academic, New York
Sharma P, Bhatti TS (2010) A review on electrochemical double-layer capacitors. Energy Convers Manag 51(12):2901–2912
Béguin F, Frąckowiak E (eds) (2013) Supercapacitors: materials, systems, and applications. Wiley, Weinheim
Ji H, Zhao X, Qiao Z, Jung J, Zhu Y, Lu Y, Zhang LL, MacDonald AH, Ruoff RS (2014) Capacitance of carbon-based electrical double-layer capacitors. Nat Commun 5(1):3317
Wang Y, Song Y, Xia Y (2016) Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem Soc Rev 45(21):5925–5950
Salanne M, Rotenberg B, Naoi K, Kaneko K, Taberna PL, Grey CP, Dunn B, Simon P (2016) Efficient storage mechanisms for building better supercapacitors. Nat Energy 1(6):16070
Zuo W, Li R, Zhou C et al (2017) Battery-supercapacitor hybrid devices: recent progress and future prospects. Adv Sci 4:1–21
Burke A (2007) R&D considerations for the performance and application of electrochemical capacitors. Electrochim Acta 53(3):1083–1091
Conte M (2010) Supercapacitors technical requirements for new applications. Fuel Cells 10(5):806–818
Kühne R (2010) Electric buses—an energy efficient urban transportation means. Energy 35(12):4510–4513
González A, Goikolea E, Barrena JA, Mysyk R (2016) Review on supercapacitors: technologies and materials. Renew Sust Energ Rev 58:1189–1206
Pandolfo AG, Hollenkamp AF (2006) Carbon properties and their role in supercapacitors. J Power Sources 157(1):11–27
Béguin F, Frąckowiak E (eds) (2010) Carbons for electrochemical energy storage and conversion systems. CRC Press, Boca Raton
Lewandowski A, Olejniczak A, Galiński M, Stępniak I (2010) Performance of carbon–carbon supercapacitors based on organic, aqueous and ionic liquid electrolytes. J Power Sources 195(17):5814–5819
Sevilla M, Mokaya R (2014) Energy storage applications of activated carbons: supercapacitors and hydrogen storage. Energy Environ Sci 7(4):1250–1280
Zhai Y, Dou Y, Zhao D, Fulvio PF, Mayes RT, Dai S (2011) Carbon materials for chemical capacitive energy storage. Adv Mater 23(42):4828–4850
Zhang J, Xiang J, Dong Z, Liu Y, Wu Y, Xu C, du G (2014) Biomass derived activated carbon with 3D connected architecture for rechargeable lithium—sulfur batteries. Electrochim Acta 116:146–151
Guo J, Zhang J, Jiang F, Zhao S, Su Q, du G (2015) Microporous carbon nanosheets derived from corncobs for lithium-sulfur batteries. Electrochim Acta 176:853–860
Titirici M-M, White RJ, Brun N, Budarin VL, Su DS, del Monte F, Clark JH, MacLachlan MJ (2015) Sustainable carbon materials. Chem Soc Rev 44(1):250–290
Galiński M, Lewandowski A, Stępniak I (2006) Ionic liquids as electrolytes. Electrochim Acta 51(26):5567–5580
Zhong C, Deng Y, Hu W, Qiao J, Zhang L, Zhang J (2015) A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem Soc Rev 44(21):7484–7539
Choudhury NA, Sampath S, Shukla AK (2009) Hydrogel-polymer electrolytes for electrochemical capacitors: an overview. Energy Environ Sci 2(1):55–67
Choudhury NA, Northrop PWC, Crothers AC, Jain S, Subramanian VR (2012) Chitosan hydrogel-based electrode binder and electrolyte membrane for EDLCs: experimental studies and model validation. J Appl Electrochem 42(11):935–943
Navarra MA, Dal Bosco C, Moreno JS et al (2015) Synthesis and characterization of cellulose-based hydrogels to be used as gel electrolytes. Membranes (Basel) 5(4):810–823
Shen X, Shamshina JL, Berton P et al (2015) Hydrogels based on cellulose and chitin: fabrication, properties, and applications. Green Chem 18:53–75
Stępniak I, Galiński M, Nowacki K et al (2016) A novel chitosan/sponge chitin origin material as a membrane for supercapacitors—preparation and characterization. RSC Adv 6(5):4007–4013
Wang H-Q, Yin J, Li Q, Yin P (2014) Current progress on the preparation of binders for electrochemical supercapacitors. Post Doc J J Postdr Res 2:31–238
Abbas Q, Pajak D, Frąckowiak E, Béguin F (2014) Effect of binder on the performance of carbon/carbon symmetric capacitors in salt aqueous electrolyte. Electrochim Acta 140:132–138
Zhu Z, Tang S, Yuan J et al (2016) Effects of various binders on supercapacitor performances. Int J Electrochem Sci 11:8270–8279
Carratalá-Abril J, Rey-Martínez L, Beneito-Ruiz R, Vilaplana-Cerdá J (2016) Development of carbon-based composite materials for energy storage. Mater Today Proc 3S:S240–S245
Dyatkin B, Presser V, Heon M, Lukatskaya MR, Beidaghi M, Gogotsi Y (2013) Development of a green supercapacitor composed entirely of environmentally friendly materials. ChemSusChem 6(12):2269–2280
Aslan M, Weingarth D, Jäckel N, Atchison JS, Grobelsek I, Presser V (2014) Polyvinylpyrrolidone as binder for castable supercapacitor electrodes with high electrochemical performance in organic electrolytes. J Power Sources 266:374–383
Aslan M, Weingarth D, Herbeck-Engel P, Grobelsek I, Presser V (2015) Polyvinylpyrrolidone/polyvinyl butyral composite as a stable binder for castable supercapacitor electrodes in aqueous electrolytes. J Power Sources 279:323–333
Khomenko V, Barsukov V, Chernysh O, Makyeyeva I, Isikli S, Gauthy F (2016) Green alternative binders for high-voltage electrochemical capacitors. IOP Conf Ser Mater Sci Eng 111:12025
Jabbour L, Bongiovanni R, Chaussy D, Gerbaldi C, Beneventi D (2013) Cellulose-based Li-ion batteries: a review. Cellulose 20(4):1523–1545
Nirmale TC, Kale BB, Varma AJ (2017) A review on cellulose and lignin based binders and electrodes: small steps towards a sustainable lithium ion battery. Int J Biol Macromol 103:1032–1043
Lux SF, Schappacher F, Balducci A, Passerini S, Winter M (2010) Low cost, environmentally benign binders for lithium-ion batteries. J Electrochem Soc 157(3):A320–A325
Hu M, Pang X, Zhou Z (2013) Recent progress in high-voltage lithium ion batteries. J Power Sources 237:229–242
Böckenfeld N, Jeong SS, Winter M, Passerini S, Balducci A (2013) Natural, cheap and environmentally friendly binder for supercapacitors. J Power Sources 221:14–20
Murashko K, Nevstrueva D, Pihlajamäki A, Koiranen T, Pyrhönen J (2017) Cellulose and activated carbon based flexible electrical double-layer capacitor electrode: preparation and characterization. Energy 119:435–441
Pérez-Madrigal MM, Edo MG, Aleman C (2016) Powering the future: application of cellulose-based materials for supercapacitors. Green Chem 18(22):5930–5956
Wang Z, Tammela P, Strømme M, Nyholm L (2017) Cellulose-based supercapacitors: material and performance considerations. Adv Energy Mater 7:1–22
Jeong SS, Böckenfeld N, Balducci A, Winter M, Passerini S (2012) Natural cellulose as binder for lithium battery electrodes. J Power Sources 199:331–335
Varzi A, Balducci A, Passerini S (2014) Natural cellulose: a green alternative binder for high voltage electrochemical double layer capacitors containing ionic liquid-based electrolytes. J Electrochem Soc 161(3):A368–A375
Xiao S, Wang F, Yang Y, Chang Z, Wu Y (2014) An environmentally friendly and economic membrane based on cellulose as a gel polymer electrolyte for lithium ion batteries. RSC Adv 4(1):76–81
Zhang J, Wu J, Yu J, Zhang X, He J, Zhang J (2017) Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: state of the art and future trends. Mater Chem Front 1(7):1273–1290
Kasprzak D, Stępniak I, Galiński M (2018) Acetate- and lactate-based ionic liquids: synthesis, characterisation and electrochemical properties. J Mol Liq 264:233–241
Acharya S, Hu Y, Moussa H, Abidi N (2017) Preparation and characterization of transparent cellulose films using an improved cellulose dissolution process. J Appl Polym Sci 134:1–12
Keiser H, Beccu KD, Gutjahr MA (1976) Abschätzung der porenstruktur poröser elektroden aus impedanzmessungen. Electrochim Acta 21(8):539–543
Suss ME, Baumann TF, Worsley MA, Rose KA, Jaramillo TF, Stadermann M, Santiago JG (2013) Impedance-based study of capacitive porous carbon electrodes with hierarchical and bimodal porosity. J Power Sources 241:266–273
Kant R, Singh MB (2017) Theory of the electrochemical impedance of mesostructured electrodes embedded with heterogeneous micropores. J Phys Chem C 121(13):7164–7174
Wei L, Yushin G (2011) Electrical double layer capacitors with sucrose derived carbon electrodes in ionic liquid electrolytes. J Power Sources 196(8):4072–4079
Tõnurist K, Jänes A, Thomberg T et al (2009) Influence of mesoporous separator properties on the parameters of electrical double-layer capacitor single cells. J Electrochem Soc 156(4):A334–A342
Eikerling M, Kornyshev AA, Lust E (2005) Optimized structure of nanoporous carbon-based double-layer capacitors. J Electrochem Soc 152(1):E24–E33
Kurig H, Vestli M, Tonurist K, Janes A, Lust E (2012) Influence of room temperature ionic liquid anion chemical composition and electrical charge delocalization on the supercapacitor properties. J Electrochem Soc 159(7):A944–A951
Pohlmann S, Olyschläger T, Goodrich P, Vicente JA, Jacquemin J, Balducci A (2015) Mixtures of Azepanium based ionic liquids and propylene carbonate as high voltage electrolytes for supercapacitors. Electrochim Acta 153:426–432
Meyers JP, Doyle M, Darling RM, Newman J (2000) The impedance response of a porous electrode composed of intercalation particles. J Electrochem Soc 147(8):2930–2940
Acknowledgements
This work was performed with the financial support of Poznan University of Technology (Grant No. 03/31/DSPB/0358/2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interests regarding the publication of this paper.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kasprzak, D., Stępniak, I. & Galiński, M. Electrodes and hydrogel electrolytes based on cellulose: fabrication and characterization as EDLC components. J Solid State Electrochem 22, 3035–3047 (2018). https://doi.org/10.1007/s10008-018-4015-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-4015-y