Abstract
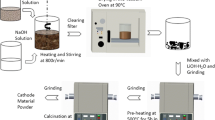
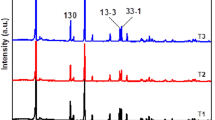
Li- and Mn-rich layered Li1.2Ni0.13Co0.13Mn0.54O2 cathode material was synthesized using sonochemical method followed by annealing at 700, 800, and 900 °C for 10 h. The material was characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscopy (TEM), Raman spectroscopy, and electrochemical techniques. Its performance as a cathode material for Li-ion batteries was examined. With the sample annealed at 900 °C, an initial specific capacity of 240 mAh g−1 was obtained, which decreased to 215 mAh g−1 after 80 cycles, thus retaining about 90 % of its initial capacity. In contrast, samples annealed at lower temperatures exhibited lower capacity retention upon cycling. Thus, the final annealing temperature was found to have a significant effect on the electrochemical stability of this material in terms of capacity, average voltage, and rate capability. The advantage of this synthesis, which includes a sonochemical stage, compared with a conventional co-precipitation synthesis, was also confirmed.








Similar content being viewed by others
References
Thackeray MM, Johnson CS, Vaughey JT, Li N, Hackney SA (2005) Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J Mater Chem 15:2257–2267
Thackeray MM, Kang SH, Johnson CS, Vaughey JT, Benedek R, Hackney SA (2007) Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater Chem 17:3112–3125
Thackeray MM, Kang SH, Johnson CS, Vaughey JT, Hackney SA (2006) Comments on the structural complexity of lithium-rich Li1+x M1-x O2 electrodes (M = Mn, Ni, Co) for lithium batteries. Electrochem Comm 8:1531–1538
Yu H, Zhou H (2012) Initial Coulombic efficiency improvement of the Li1.2Mn0.567Ni0.166Co0.067O2 lithium-rich material by ruthenium substitution for manganese. J Mater Chem 22:15507–15510
Amalraj F, Kovacheva D, Talianker M, Zeiri L, Grinblat J, Leifer N, Goobes G, Markovsky B, Aurbach D (2010) Synthesis of Integrated Cathode Materials xLi2MnO3⋅ (1 − x) LiMn1/3Ni1/3Co1/3O2 (x = 0.3, 0.5, 0.7) and Studies of Their Electrochemical Behavior. J Electrochem Soc 157:A1121–A1130
Li J, Klopsch R, Stan MC, Nowak S, Kunze M, Winter M, Passerini S (2011) Synthesis and electrochemical performance of the high voltage cathode material Li[Li0.2Mn0.56Ni0.16Co0.08]O2 with improved rate capability. J Power Sources 196:4821–4825
Amalraj F, Talianker M, Markovsky B, Sharon D, Burlaka L, Shafir G, Zinigrad E, Haik O, Aurbach D, Lampert J, Dobrick MS, Garsuch A (2013) Study of the lithium-rich integrated compound xLi2MnO3·(1-x)LiMO2 (x around 0.5; M = Mn, Ni, Co; 2:2:1) and its electrochemical activity as positive electrode in lithium cells. J Electrochem Soc 160:A324–A337
Penki TR, Shanmughasundaram D, Jeyaseelan AV, Subramani AK, Munichandraiah N (2014) Polymer template assisted synthesis of porous Li1.2Mn0.53Ni0.13Co0.13O2 as a high capacity and high rate capability positive electrode material. J Electrochem Soc 161:A33–A39
Nayak PK, Grinblat J, Levi M, Markovsky B, Wu Y, Powell B, Aurbach D (2014) Structural and electrochemical evidence of layered to spinel phase transformation of Li and Mn rich layered cathode materials of the formulae xLi[Li1/3Mn2/3]O2.(1-x)LiMn1/3Ni1/3Co1/3O2 (x = 0.2, 0.4, 0.6) upon cycling. J Electrochem Soc 161:A1534–A1547
Nayak PK, Grinblat J, Levi M, Aurbach D (2014) Electrochemical and structural characterization of carbon coated Li1.2Mn0.56Ni0.16Co0.08O2 and Li1.2Mn0.6Ni0.2O2 as cathode materials for Li-ion batteries. Electrochim Acta 137:546–556
Yabuuchi N, Yoshii K, Myung ST, Nakai I, Komaba S (2011) Detailed studies of a high-capacity electrode material for rechargeable batteries Li2MnO3-LiCo1/3Ni1/3Mn1/3O2. J Am Chem Soc 133:4404–4419
Liu H, Du C, Yin G, Song B, Zuo P, Cheng X, Ma Y, Gao Y (2014) An Li-rich oxide cathode material with mosaic spinel grain and a surface coating for high performance Li-ion batteries. J Mater Chem A 2:15640–15646
Elia GA, Wang J, Bresser D, Li J, Scrosati B, Passerini S, Hassoun J (2014) A new, high energy Sn−C/Li[Li0.2Ni0.4/3Co0.4/3Mn1.6/3]O2 lithium-ion battery. ACS Appl Mater Interfaces 6:12956–12961
Wang Z, Liu E, He C, Shi C, Li J, Zhao N (2013) Effect of amorphous FePO4 coating on structure and electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 as cathode material for Li-ion batteries. J Power Sources 236:25–32
Zheng JM, Wu XB, Yang Y (2011) A comparison of preparation method on the electrochemical performance of cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 for lithium ion battery. Electrochim Acta 56:3071–3078
Yang S, Huang G, Hu S, Hou X, Huang Y, Yue M, Lei G (2014) Improved electrochemical performance of the Li1.2Ni0.13Co0.13Mn0.54O2 wired by CNT networks for lithium-ion batteries. Mater Lett 118:8–11
Li Q, Li G, Fu C, Luo D, Fan J, Li L (2014) K+-Doped Li1.2Mn0.54Co0.13Ni0.13O2: a novel cathode material with an enhanced cycling stability for lithium-ion batteries. ACS Appl Mater Interfaces 6:10330–10341
Shi SJ, Tu JP, Tang YY, Zhang YQ, Wang XL, Gu CD (2013) Preparation and characterization of macroporous Li1.2Mn0.54Ni0.13Co0.13O2 cathode material for lithium-ion batteries via aerogel template. J Power Sources 240:140–148
Shi SJ, Tu JP, Tang YY, Liu XY, Zhang YQ, Wang XL, Gu CD (2013) Enhanced cycling stability of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by surface modification of MgO with melting impregnation method. Electrochim Acta 88:671–679
West WC, Soler J, Smart MC, Ratnakumar BV, Firdosy S, Ravi V, Anderson MS, Hrbacek J, Lee ES, Manthiram A (2011) Electrochemical behavior of layered solid solution Li2MnO3 − LiMO2 (M = Ni, Mn, Co) Li-ion cathodes with and without alumina coatings. J Electrochem Soc 158:A883–A889
Li GR, Feng X, Ding Y, Ye SH, Gao XP (2012) AlF3-coated Li(Li0.17Ni0.25Mn0.58)O2 as cathode material for Li-ion batteries. Electrochim Acta 78:308–315
Amalraj F, Talianker M, Markovsky B, Burlaka L, Leifer N, Goobes G, Erickson EM, Haik O, Grinblat J, Zinigrad E, Aurbach D, Lampert JK, Shin JY, Dobrick MS, Garsuch A (2013) Studies of Li and Mn-rich Lix[MnNiCo]O2 electrodes: electrochemical performance, structure, and the effect of the aluminum fluoride coating. J Electrochem Soc 160:A2220–A2233
Wang D, Huang Y, Huo Z, Chen L (2013) Synthesize and electrochemical characterization of Mg-doped Li-rich layered Li[Li0.2Ni0.2Mn0.6]O2 cathode material. Electrochim Acta 107:461–466
Jina X, Xua Q, Liua H, Yuana X, Xia Y (2014) Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery. Electrochim Acta 136:19–26
Li Z, Chernova NA, Feng J, Upreti S, Omenya F, Whittingham MS (2012) Stability and rate capability of Al substituted lithium-rich high-manganese content oxide materials for Li-ion batteries. J Electrochem Soc 159:A116–A120
Lee E, Koritala R, Miller DJ, Johnson CS (2015) Aluminum and gallium substitution into 0.5Li2MnO3·0.5Li(Ni0.375Mn0.375Co0.25)O2 layered composite and the voltage fade effect. J Electrochem Soc 162:A322–A329
Nayak PK, Grinblat J, Levi M, Haik O, Levi E, Aurbach D (2015) Effect of Fe in suppressing the discharge voltage decay of high capacity Li-rich cathodes for Li-ion batteries. J Solid State Electrochem 19:2781–2792
Nayak PK, Grinblat J, Levi M, Aurbach D (2015) Understanding the effect of lithium bis(oxalato) borate (LiBOB) on the structural and electrochemical aging of Li and Mn rich high capacity Li1.2Ni0.16Mn0.56Co0.08O2 cathodes. J Electrochem Soc 162:A596–A602
Lv W, Qiu Q, Wang F, Wei S, Liu B, Luo Z (2010) Sonochemical synthesis of cobalt aluminate nanoparticles under various preparation parameters. Ultrason Sonochem 17:793–801
Malka E, Perelshtein I, Lipovsky A, Shalom Y, Naparstek L, Perkas N, Patick T, Lubart R, Nitzan Y, Banin E, Gedanken A (2013) Eradication of multi-drug resistant bacteria by a novel Zn-doped CuO nanocomposite. Small 9:4069–4076
Friedman H, Reich S, Popovitz-Biro R, von Huth P, Halevy I, Koltypin Y, Gedanken A, Porat Z (2013) Micro- and nano-spheres of low melting point metals and alloys, formed by ultrasonic cavitation. Ultrason Sonochem 20:432–444
Soltani T, Entezari MH (2013) Sono-synthesis of bismuth ferrite nanoparticles with high photocatalytic activity in degradation of Rhodamine B under solar light irradiation. Chem Engg Journal 223:145–154
Yuvaraj S, Selvan RK, Kumar VB, Perelshtein I, Gedanken A, Isakkimuthu S, Arumugam S (2014) Sonochemical synthesis, structural, magnetic and grain size dependent electrical properties of NdVO4 nanoparticles. Ultrason Sonochem 21:599–605
Sivakumar P, Nayak PK, Markovsky B, Aurbach D, Gedanken A (2015) Sonochemical synthesis of LiNi0.5Mn1.5O4 and its electrochemical performance as a cathode material for 5 V Li-ion batteries. Ultrason Sonochem 26:332–339
Xiang Y, Yin Z, Li X (2014) An improved carbonate precipitation method for the preparation of Li1.2Ni0.12Co0.12Mn0.56O2 cathode material. Ionics 20:163–168
Xue Y, Wang Z, Yu F, Zhang Y, Yin G (2014) Ethanol-assisted hydrothermal synthesis of LiNi0.5Mn1.5O4 with excellent long-term cyclability at high rate for lithium-ion batteries. J Mater Chem A 2:4185–4191
Ohayon E, Gedanken A (2010) The application of ultrasound radiation to the synthesis of nanocrystalline metal oxide in a non-aqueous solvent. Ultrason Sonochem 17:173–178
Som S, Kumar V, Kumar V, Gohain M, Pandey A, Duvenhagea MM, Terblans JJ, Bezuindenhoud BCB, Swart HC (2016) Dopant distribution and influence of sonication temperature on the pure red light emission of mixed oxide phosphor for solid state lighting. Ultrason Sonochem 28:79–89
Vijayakumar R, Koltypin Y, Felner I, Gedanken A (2000) Sonochemical synthesis and characterization of pure nanometer-sized Fe3O4 particles. Materials Science Engineering: A 286:101–105
Pinjari DV, Pandit AB (2011) Room temperature synthesis of crystalline CeO2 nanopowder: advantage of sonochemical method over conventional method. Ultrason Sonochem 18:1118–1123
Liu J, Hou M, Yi J, Guo S, Wang C, Xia Y (2014) Improving the electrochemical performance of layered lithium-rich transition-metal oxides by controlling the structural defects. Energy Environ Sci 7:705–714
Armstrong AR, Holzapfel M, Novak P, Johnson CS, Kang SH, Thackeray MM, Bruce PG (2006) Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J Am Chem Soc 128:8694–8698
Chen Y, Chen Z, Xie K (2014) Effect of annealing on the first-cycle performance and reversible capabilities of lithium-rich layered oxide cathodes. J Phys Chem C 118:11505–11511
Acknowledgments
P. Sivakumar thanks the Council for Higher Education, the State of Israel, for the PBC scholarship for outstanding postdoctoral researchers from China and India. This work was partially supported by the Israel Science Foundation-ISF, as part of the INREP project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivakumar, P., Nayak, P.K., Grinblat, J. et al. Effect of sonochemistry: Li- and Mn-rich layered high specific capacity cathode materials for Li-ion batteries. J Solid State Electrochem 20, 1683–1695 (2016). https://doi.org/10.1007/s10008-016-3176-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3176-9