Abstract
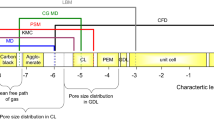
In this work, a new strategy is adopted to optimize the cathode catalyst layer of a PEM fuel cell and to analyse the impact of three pertinent design variables. A pore scale, three-dimensional model is developed to predict the performance of a single agglomerate of the catalyst layer. The pore scale modelling is implemented as a two-step procedure. First, the microstructure of the catalyst layer is computationally reconstructed and then the governing and constitutive equations can be discretized and numerically solved. The agglomerate reconstruction follows a stochastic approach of the catalyst layer structure, and random routines are added to capture the variability and imperfections of the fabrication methods. From the obtained results, it is concluded that the design variables with the highest impact on performance were the carbon particle diameter and the Nafion volume fraction. In contrast, the agglomerate diameter did not show any impact on performance for the tested operational conditions. Another important finding was related with the influence of the operational pressure on the design variables performance impact. As pressure increased, the design variables contribution to the potential variability vanished. This work also demonstrated that performance was markedly affected by the random nature of the agglomerate structure.













Similar content being viewed by others
Abbreviations
- a c :
-
Specific surface area, m2
- A m :
-
Area of the interface porous/Nafion, m2
- c :
-
Concentration, mol m−3
- c 0 :
-
Concentration at the porous/Nafion interface, mol m−3
- \( {c}_{O_2}^{ref} \) :
-
Reference concentration, mol m−3
- \( \tilde{D} \) :
-
Binary diffusivity, m s−1
- D m :
-
Diffusion coefficient in Nafion, m s−1
- DOF p :
-
Degrees of freedom
- E :
-
Activation energy, J
- E rev :
-
Reversible potential, V
- F :
-
Faraday’s constant, C mol−1
- F CF :
-
F-value for each control factor
- F critical :
-
F critical
- I :
-
Current, A
- i :
-
Current density, A m−2
- i 0 :
-
Exchange current density, A m−2
- j m :
-
Mass flux, kg m−2 s−1
- j n :
-
Molar flux, mol m−2 s−1
- L :
-
Number of levels per control factor
- L c :
-
Catalyst loading, kg m−2
- N :
-
Number of simulations
- M :
-
Molecular mass, kg mol−1
- n :
-
Number of electrons
- p :
-
Pressure, Pa
- \( {p}_{H_2O} \) :
-
Water partial pressure, Pa
- R :
-
Gas constant, J K−1 mol−1
- \( {S}_{O_2} \) :
-
Oxygen solubility in Nafion, mol m−3 Pa−1
- SS e :
-
Sum of squared error
- SS p :
-
Sum of squared deviations
- SS T :
-
Sum of squares
- T :
-
Temperature, K
- U :
-
Potential, V
- V p :
-
Variance of each control factor
- w :
-
Mass fraction
- x :
-
Molar fraction
- α :
-
Charge transfer coefficient
- γ :
-
Pressure coefficient or reaction order
- η :
-
Overpotential, V
- λ :
-
Water uptake
- ρ :
-
Species mixture density, kg m−3
- i and j :
-
Chemical species
References
Barbir F (2005) PEM fuel cells theory and practice, 1st edn. Elsevier Academic Press, Burlington
Martin S, Martinez-Vazquez B, Garcia-Ybarra PL, Castillo JL (2013) Peak utilization of catalyst with ultra-low Pt loaded PEM fuel cell electrodes prepared by the electrospray method. J Power Sources 229:179–184
Hwang DS, Park CH, Yi SC, Lee YM (2011) Optimal catalyst layer structure of polymer electrolyte membrane fuel cell. Int J Hydrog Energy 36:9876–9885
Wang Q, Song D, Navessin T et al (2004) A mathematical model and optimization of the cathode catalyst layer structure in PEM fuel cells. Electrochim Acta 50:725–730
Wu J, Liu Q, Fang H (2006) Toward the optimization of operating conditions for hydrogen polymer electrolyte fuel cells. J Power Sources 156:388–399
Hattori T, Suzuki A, Sahnoun R et al (2008) Development of the overpotential simulator for polymer electrolyte fuel cells and application for optimization of cathode structure. Appl Surf Sci 254:7929–7932
Zervas PL, Tatsis A, Sarimveis H, Markatos NCG (2008) Development of a novel computational tool for optimizing the operation of fuel cells systems: application for phosphoric acid fuel cells. J Power Sources 185:345–355
Srinivasarao M, Bhattacharyya D, Rengaswamy R, Narasimhan S (2011) Multivariable optimization studies of cathode catalyst layer of a polymer electrolyte membrane fuel cell. Chem Eng Res Des 89:10–22
Khajeh-Hosseini-Dalasm N, Fesanghary M, Fushinobu K, Okazaki K (2012) A study of the agglomerate catalyst layer for the cathode side of a proton exchange membrane fuel cell: modeling and optimization. Electrochim Acta 60:55–65
Srinivasarao M, Bhattacharyya D, Rengaswamy R (2012) Optimization studies of a polymer electrolyte membrane fuel cell with multiple catalyst layers. J Power Sources 206:197–203
Xing L, Song X, Scott K et al (2013) Multi-variable optimisation of PEMFC cathodes based on surrogate modelling. Int J Hydrog Energy 38:14295–14313
Kopanidis A, Theodorakakos A, Gavaises M, Bouris D (2011) Pore scale 3D modelling of heat and mass transfer in the gas diffusion layer and cathode channel of a PEM fuel cell. Int J Therm Sci 50:456–467
Lange KJ, Sui P, Djilali N (2011) Pore scale modeling of a proton exchange membrane fuel cell catalyst layer : effects of water vapor and temperature. J Power Sources 196:3195–3203
Lange KJ, Sui P, Djilali N (2012) Determination of effective transport properties in a PEMFC catalyst layer using different reconstruction algorithms. J Power Sources 208:354–365
Wu R, Liao Q, Zhu X, Wang H (2012) Pore network modeling of cathode catalyst layer of proton exchange membrane fuel cell. Int J Hydrog Energy 37:11255–11267
Salomov UR, Chiavazzo E, Asinari P (2014) Pore-scale modeling of fluid flow through gas diffusion and catalyst layers for high temperature proton exchange membrane (HT-PEM) fuel cells. Comput Math Appl 67:393–411
Salomov UR, Chiavazzo E, Asinari P (2015) Gas-dynamic and electro-chemical optimization of catalyst layers in high temperature polymeric electrolyte membrane fuel cells. Int J Hydrog Energy 40:5425–5431
Sousa T, Mamlouk M, Scott K (2010) An isothermal model of a laboratory intermediate temperature fuel cell using PBI doped phosphoric acid membranes. Chem Eng Sci 65:2513–2530
Sousa T, Mamlouk M, Scott K (2010) A non-isothermal model of a laboratory intermediate temperature fuel cell using PBI doped phosphoric acid membranes. Fuel Cells 10:993–1012
Sousa T, Mamlouk M, Scott K (2010) A dynamic non-isothermal model of a laboratory intermediate temperature fuel cell using PBI doped phosphoric acid membranes. Int J Hydrog Energy 35:12065–12080
Mamlouk M, Sousa T, Scott K (2011) A high temperature polymer electrolyte membrane fuel cell model for reformate gas. Int J Electrochem 2011:1–18
Sousa T, Mamlouk M, Scott K, Rangel CM (2012) Three dimensional model of a high temperature PEMFC. Study of the flow field effect on performance. Fuel Cells 12:566–576
Zhang F-Y, Spernjak D, Prasad AK, Advan SG (2007) In situ characterization of the catalyst layer in a polymer electrolyte membrane fuel cell. J Electrochem Soc 154:B1152–B1156
Yim S-D, Sohn Y-J, Park S-H et al (2011) Fabrication of microstructure controlled cathode catalyst layers and their effect on water management in polymer electrolyte fuel cells. Electrochim Acta 56:9064–9073
Qi Z, Kaufman A (2003) Low Pt loading high performance cathodes for PEM fuel cells. J Power Sources 113:37–43
Sasikumar G, Ihm JW, Ryu H (2004) Dependence of optimum Nafion content in catalyst layer on platinum loading. J Power Sources 132:11–17
Ross PJ (1995) Taguchi techniques for quality engineering, 2nd edn. McGraw-Hill, New York
Acknowledgments
The present work is financed by FEDER funds through Programa Operacional Factores de Competitividade—COMPETE and by national funds through the Fundação para a Ciência e a Tecnologia (FCT) Project FCOMP-01-0124-FEDER-028580.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
Table 6 shows the 27 simulations with the corresponded levels of the design variables. These simulations were used to analyse the influence of each design variable level on performance.
Appendix B. Analysis of variance (ANOVA)
The analysis of variance (ANOVA) is of particular interest to identify the relative importance amongst the analysed control factors on performance, and also to decide, through the F-test, if a specific control factor is significant or not for the performance variability.
In ANOVA, the analysis of variance is achieved by separating the total variability of the S/N ratios into contributions of each control factor and error. The total variability was obtained by the sum of the squared deviations from the total mean S/N ratio:
where, SS T is the sum of squares, N is the number of simulations in the orthogonal array (N = 18), S/N i is the S/N value for the i th case and \( \overline{S/N} \) is the total mean of the S/N values.
The contribution of each control factor to the sum of squares was calculated as:
where, SS p is the sum of squared deviations due to each control factor, j is the level number, L is the number of levels per control factor, t is the repetition per level and sS/N j is the sum of the S/N ratio involving level j. The sum of squared error (SS e ) was then easily derived from the sum of squares and from the sum of the squared deviation of each control factor. The variance of the control factor was simply:
where, V p is the variance of each control factor or mean square deviation and DOF p are the degrees of freedom. The F-value for each control factor (F CF ) was simply the ratio of the mean of squares deviations to the mean of the squared error. Finally, the percentage of contribution by each of the control factor in the total sum of squared deviations was the ratio between SS p and SS T . More details about this methodology can be found elsewhere [27].
The F critical (F critical ) was found based on the statistical approach which obeyed f-distribution with L-1 numerator degrees of freedom, N-L denominator degrees of freedom and a significance level of 0.05. Having into account these considerations the F critical was found and the hypothesis for accepting or rejecting the significance of a control factor was given by the following rules: the control factor was not significant if F CF ≤ F critical ; the control factor was significant if F CF > F critical .
Rights and permissions
About this article
Cite this article
Sousa, T., Rangel, C.M. Pore scale modelling of a cathode catalyst layer in fuel cell environment: agglomerate reconstruction and variables optimization. J Solid State Electrochem 20, 541–554 (2016). https://doi.org/10.1007/s10008-015-3076-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-3076-4