Abstract
Context
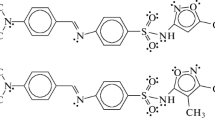
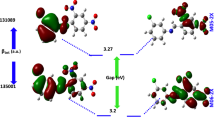
The present work deals with the linear and nonlinear optical properties such as the dipole moment, polarizability, total hyperpolarizability, electric field–induced second harmonic generation, and hyper-Rayleigh scattering first hyperpolarizability of four heterocyclic azo compounds containing the sulfonamide group considered promise in nonlinear optic. The obtained polarizability and hyperpolarizability were supported by the frontier molecular orbital analysis. The properties have been effectively estimated and thoroughly examined to shed light on the nonlinear optical activity based on the density functional theory. The observed results show a high total first hyperpolarizability \({\beta }_{{\text{tot}}}\) up to 2503 a.u. and a low energy gap \({E}_{{\text{g}}}\) less than 1.41 eV. An inverse relationship has been obtained between the \({\beta }_{{\text{tot}}}\) and \({E}_{{\text{g}}}\). The calculated \({E}_{{\text{g}}}\) values confirm that charge occurs within the azo sulfonamides. The new study provides a promising avenue for the development of these azo sulfonamides as novel NLO materials.
Methods
The molecular modeling and the theoretical studies were performed with Gaussian software packages. The B3LYP/6–311 + G** level was used for optimization. All the linear and nonlinear optical properties reported here are obtained using the DFT. The optimized structures and their frontier molecular orbitals were plotted using the GaussView 5.1 program.











Similar content being viewed by others
Data availability
All data generated or analyzed during this study, which support the plots within this paper and the other findings of this study, are included in this article and its supplementary information. Source data are provided in this paper.
References
Rouhani Sh, Hosseinnezhad M (2015) Application of azo dye as sensitizer in dye-sensitized solar cells. Prog Color Colorants Coat 8:259–265. https://doi.org/10.30509/PCCC.2015.75864
Eltaboni R, Bader N, Elsharif R, Ahmida A (2022) Chemistry and applications of azo dyes: a comprehensive review. J Chem Rev 4:313–330. https://doi.org/10.22034/jcr.2022.349827.1177
Yazdanbakhsh MR, Mohammadi A, Abbasnia M (2010) Some heterocyclic azo dyes derived from thiazolyl derivatives; synthesis; substituent effects and solvatochromic studies. Spectrochim Acta Part A Mol Biomol Spectrosc 77:1084–1087. https://doi.org/10.1016/j.saa.2010.08.079
Zhang Y, Yang H, Sun Y, Zheng X, Guo (2022) Molecular dynamics simulations on photoinduced switchable Tg and self-healing behaviors of azobenzene-containing polymers. Comput Mater Sci 215:111810. https://doi.org/10.1016/j.commatsci.2022.111810
Mikroyannidis JA, Tsagkournos DV, Balraju P, Sharma GD (2011) Low band gap dyes based on 2-styryl-5-phenylazo-pyrrole: synthesis and application for efficient dye-sensitized solar cells. J Power Sources 196:4152–4161. https://doi.org/10.1016/j.jpowsour.2010.12.038
Delaire JA, Nakatani K (2000) Linear and nonlinear optical properties of photochromic molecules and materials. Chem Rev 100:1817–1846. https://doi.org/10.1021/cr980078m
Fominykh OD, Sharipova AV, Balakina MY (2019) Atomistic modeling of polymer materials based on methacrylic copolymers with azochromophores in the side chain. Comput Mater Sci 168:32–39. https://doi.org/10.1016/j.commatsci.2019.05.057
Raposo MMM, Ferreira AMFP, Amaro M, Belsley M, Moura CVP (2009) The synthesis and characterization of heterocyclic azo dyes derived from 5-N, N-dialkylamino-2,2’-bithiophene couplers. Dyes Pigm 83:59–65. https://doi.org/10.1016/j.dyepig.2009.03.012
Derkowska-Zielinska B, Gondek E, Pokladko-Kowar M, Kaczmarek-Kedziera A, Kysil A, Lakshminarayana G, Krupka O (2020) Photovoltaic cells with various azo dyes as components of the active layer. Sol Energy 203:19–24. https://doi.org/10.1016/j.solener.2020.04.022
Fominykh OD, Sharipova AV, Balakina MY (2022) Molecular modeling of methacrylic composite materials doped with nonlinear optical azochromophores with various acceptor fragments. Comput Mater Sci 201:110909. https://doi.org/10.1016/j.commatsci.2021.110909
Polymers D, Jánossy I, Tóth-katona T (2022) Photo-orientation of liquid crystals on azo dye-containing polymers, István Jánossy and Tibor Tóth-Katona. Polymers 14:159. https://doi.org/10.3390/polym14010159
Belmar J (2010) New liquid crystals containing the benzothiazol unit: amides and azo compounds. Liq Cryst 26:389–396. https://doi.org/10.1080/026782999205164
Na H, Kim J, Hong K, Ko B (2006) Synthesis of azo dye containing polymers and application for optical data storage. Mol Cryst Liq 349:35–38. https://doi.org/10.1080/10587250008024859
Athira LS, Balachandran S, Annaraj J, Noelson EA (2019) Molecular structure, spectroscopic, solvatochromic, dyeing performance and biological evaluations of heterocyclic azo dye, 4-[(E)-(4-hydroxy-2-methylphenyl)diazenyl]-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one. J Mol Struct 1195:556–569. https://doi.org/10.1016/j.molstruc.2019.06.007
Yadav SB, Taware S, Sreenath MC, Chitrambalam S, Joe IH, Sekar N (2020) Experimental and theoretical investigation of linear and nonlinear optical properties of ethyl-3-hydroxy-2-napthoate azo dyes by solvatochromic, computational aspects, and Z-scan technique. J Phys Org Chem 33:4050. https://doi.org/10.1002/poc.4050
Coelho PJ, Carvalho LM, Moura JCVP, Raposo MMM (2009) Novel photochromic 2, 2′-bithiophene azo dyes. Dyes Pigm 82:130–133. https://doi.org/10.1016/j.dyepig.2008.12.005
Juvekar V, Lim CS, Lee DJ, Park SJ, Song GO, Kang H, Kim HM (2021) An azo dye for photodynamic therapy that is activated selectively by two-photon excitation. Chem Sci 12:427–434. https://doi.org/10.1039/d0sc05686c
Sultan HA, Muala A, Hassan QMA, Fahad T, Emshary CA, Raheem NA (2021) Synthesis, characterization and the nonlinear optical properties of newly benzenesulfonamide. Spectrochim Acta Part A Mol Biomol Spectrosc 251:119487. https://doi.org/10.1016/j.saa.2021.119487
Djeukoua KSD, Fondjo ES, Tamokou J, Tsemeugne J, Peter FW, Tsopmo A, Tchieno FMM, Ekom SE, Pecheu CN, Tonle IK, Kuiateb J-R (2019) Synthesis, characterization, antimicrobial activities and electrochemical behavior of new phenolic azo dyes from two thienocoumarin amines. Arkivoc 416–430. https://doi.org/10.24820/ark.5550190.p010.994
El-Sonbati AZ, Diab MA, Morgan SM (2019) Thermal properties, antimicrobial activity and DNA binding of Ni (II) complexes of azo dye compounds. J Mol Liq 225:195–206. https://doi.org/10.1016/j.molliq.2016.11.047
Tahir T, Ashfaq M, Saleem M, Rafiq M, Shahzad MI, Kotwica-Mojzych K, Mojzych M (2021) Pyridine scaffolds, phenols and derivatives of azo moiety: current therapeutic perspectives. Molecules 26:4872. https://doi.org/10.3390/molecules26164872
Gupta VK, Saravanan R, Agarwal S, Gracia F, Khan MM, Qin J, Mangalaraja RV (2017) Degradation of azo dyes under different wavelengths of UV light with chitosan-SnO2 nanocomposites. J Mol Liq 232:423–430. https://doi.org/10.1016/j.molliq.2017.02.095
Saeed AM, Alneyadi SS, Abdou IM, Access O (2020) Anticancer activity of novel Schiff bases and azo dyes derived from 3-amino-4-hydroxy-2H-pyrano [3,2-c] quinoline-2,5 (6H)-dione. Heterocycl Comm 26:192–205. https://doi.org/10.1515/hc-2020-0116
El-ghamry HA, Al-ziyadi RO, Alkhatib FM, Takroni KM, Khedr AM (2023) Metal chelates of sulfafurazole azo dye derivative: synthesis, structure affirmation, antimicrobial, antitumor, DNA binding, and molecular docking simulation. Bioinorg Chem Appl 2023:2239976. https://doi.org/10.1155/2023/2239976
Janjua MRSA, Jamil S, Mahmood A, Zafar A, Haroon M, Bhatti HN (2015) Solvent-dependent non-linear optical properties of 5,5′-disubstituted-2,2′-bipyridine complexes of ruthenium(II): a quantum chemical perspective. Aust J Chem 68:1502–1507. https://doi.org/10.1071/CH14736
Janjua MRSA, Mahmood A, Ahmad F (2013) Solvent effects on nonlinear optical response of certain tetrammineruthenium(II) complexes of modified 1,10-phenanthrolines. Can J Chem 91:1303–1309. https://doi.org/10.1139/cjc-2013-0377
Janjua MRSA (2021) Quantum chemical design of D–π–A-type donor materials for highly efficient, photostable, and vacuum-processed organic solar cells. Energy Technol 9:1–12. https://doi.org/10.1002/ente.202100489
Janjua MRSA (2021) Theoretical framework for encapsulation of inorganic B12N12 nanoclusters with alkaline earth metals for efficient hydrogen adsorption: a step forward toward hydrogen storage materials. Inorg Chem 60:2816–2828. https://doi.org/10.1021/acs.inorgchem.0c03730
Janjua MRSA, Su Z-M, Guan W, Liu C-G, Yan L-K, Song P, Maheen G (2010) Tuning second-order non-linear (NLO) optical response of organoimido-substituted hexamolybdates through halogens: quantum design of novel organic-inorganic hybrid NLO materials. Aust J Chem 63:836. https://doi.org/10.1071/CH10094
Janjua MRSA, Guan W, Yan L, Su Z-M, Karim A, Akbar J (2010) Quantum chemical design for enhanced second-order NLO response of terpyridine-substituted hexamolybdates. Eur J Inorg Chem 2010:3466–3472. https://doi.org/10.1002/ejic.201000428
Janjua MRSA, Guan W, Yan L, Su Z-M, Ali M, Bukhari IH (2010) Prediction of robustly large molecular second-order nonlinear optical properties of terpyridine-substituted hexamolybdates: structural modelling towards a rational entry to NLO materials. J Mol Graph Model 28:735–745. https://doi.org/10.1016/j.jmgm.2010.01.011
Janjua MRSA, Irfan A, Hussien M, Ali M, Saqib M, Sulaman M (2022) Machine-learning analysis of small-molecule donors for fullerene based organic solar cells. Energy Technol 10:2200019. https://doi.org/10.1002/ente.202200019
Janjua MRSA (2017) Nonlinear optical response of a series of small molecules: quantum modification of π-spacer and acceptor. J Iran Chem Soc 14:2041–2054. https://doi.org/10.1007/s13738-017-1141-x
Kim T, Lee K (2015) D-π-A conjugated molecules for optoelectronic applications. Macromol Rapid Commun 36:943–958. https://doi.org/10.1002/marc.201400749
Dennington R, Keith T, Millam JM (2009) GaussView version 5, SemichemInc., Shawnee Mission, KS
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627. https://doi.org/10.1021/j100096a001
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158–6170. https://doi.org/10.1063/1.478522
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57. https://doi.org/10.1016/j.cplett.2004.06.011
Chai J-D, Head-Gordon M (2008) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys 128:084106. https://doi.org/10.1063/1.2834918
Zhao Y, Truhlar DG (2006) Density functional for spectroscopy: no long-range self-interaction error, good performance for Rydberg and charge-transfer states, and better performance on average than B3LYP for ground states. J Phys Chem A 110:13126–13130. https://doi.org/10.1021/jp066479k
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JJE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision C.01. Gaussian, Inc., Wallingford, CT
Hadji D, Bousmaha K, Boumediene M (2023) NLO azo compounds with sulfonamide groups: a theoretical investigation. J Indian Chem Soc 100:101062. https://doi.org/10.1016/j.jics.2023.101062
Bishop DM, Norman P (2001) Chapter 1 -Calculations of dynamic hyperpolarizabilities for small and medium-sized molecules. Handb Adv Electron Photonic Mater Devices 9:1–62. https://doi.org/10.1016/B978-012513745-4/50072-X
Bersohn R, Yoh-Han PAO, Frisch HL (1966) Double-quantum light scattering by molecules. J Chem Phys 45:3184–3198. https://doi.org/10.1063/1.1728092
Almashal F, Jabar AM, Dhumad AM (2018) Synthesis, characterization and DFT computational studies of new heterocyclic azo compounds. Eur J Chem 9:84–88. https://doi.org/10.5155/eurjchem.9.2.84-88.1683
Technology D, Technology C, Marg NP, Mh M (2018) Substituent effects on linear and nonlinear optical properties of fluorescent (E)-2-(4-halophenyl)-7-arlstyrylimidazo[1,2-A] pyridine: spectroscopic and computational methods. Phys Sci Rev 4:20180032. https://doi.org/10.1515/psr-2018-0032
Tessore F, Di Carlo G, Forni A, Righetto S, Limosani F, Biroli O A (2020) Second order nonlinear optical properties of 4-styrylpyridines axially coordinated to A4 ZnII porphyrins: a comparative experimental and theoretical investigation. Inorganics 8:45. https://doi.org/10.3390/inorganics8080045
Guezguez I, Ayadi A, Ordon K, Iliopoulos K, Branzea DG, Migalska-Zalas A, Makowska-Janusik M, El-Ghayoury A, Sahraoui B (2014) Zinc induced a dramatic enhancement of the nonlinear optical properties of an azo-based iminopyridine ligand. J Phys Chem C 118:7545–7553. https://doi.org/10.1021/jp412204f
Haroon M, Khalid M, Shafiq Z, Khan MU, Janjua MRSA (2021) High-throughput calculations and experimental insights towards the development of potent thiazoline based functional materials. Mater Today Commun 27:102485. https://doi.org/10.1016/j.mtcomm.2021.102485
Prabavathi N, Nayaki NS, Reddy BV (2015) Molecular structure, vibrational spectra, natural bond orbital and thermodynamic analysis of 3,6-dichloro-4-methylpyridazine and 3,6-dichloropyridazine-4-carboxylic acid by dft approach. Spectrochim Acta A Mol Biomol Spectrosc 136B:1134–1148. https://doi.org/10.1016/j.saa.2014.09.137
Abdel-Kader NS, Abdel-Latif SA, El-Ansary AL, Sayed AG (2021) Spectroscopic studies, density functional theory calculations, non-linear optical properties, biological activity of 1-hydroxy-4-((4-(N-(pyrimidin-2-yl)sulfamoyl)phenyl)diazenyl)-2-naphthoic acid and its chelates with nickel (II), copper (II), zinc (II) metal ions. J Mol Struct 1223:129203. https://doi.org/10.1016/j.molstruc.2020.129203
Shahid M, Salim M, Khalid M, Tahir MN, Khan MU, Braga AAC (2018) Synthetic, XRD, non-covalent interactions and solvent dependent nonlinear optical studies of Sulfadiazine-Ortho-Vanillin Schiff base: (E)-4-((2-hydroxy-3-methoxy-benzylidene) amino)-N-(pyrimidin-2-yl)benzene-sulfonamide. J Mol Struct 1161:66–75. https://doi.org/10.1016/j.molstruc.2018.02.043
Özdemir N, Dayan S, Dayan O, Dinçer M, Kalaycıoğlu NÖ (2013) Experimental and molecular modeling investigation of (E)-N-{2-[(2-hydroxybenzylidene)amino]phenyl}benzenesulfonamide. Mol Phys 111:707–723. https://doi.org/10.1080/00268976.2012.742209
Chandran A, Varghese HT, Mary YS, Panicker CY, Manojkumar TK, Van Alsenoy C, Rajendran G (2012) FT-IR, FT-Raman and computational study of (E)-N-carbamimidoyl-4-((4-methoxybenzylidene)amino)benzenesulfonamide. Spectrochim Acta A Mol Biomol Spectrosc 92:84–90. https://doi.org/10.1016/j.saa.2012.02.030
Procházková E, Čechová L, Kind J, Janeba Z, Thiele CM, Dračínský M (2018) Photoswitchable intramolecular hydrogen bonds in 5-phenylazopyrimidines revealed by in situ irradiation NMR spectroscopy. Chem - Eur J 24:492–498. https://doi.org/10.1002/chem.201705146
Gieseking B, Jäck B, Preis E, Jung S, Forster M, Scherf U, Deibel C, Dyakonov V (2012) Excitation dynamics in low band gap donor–acceptor copolymers and blends. Adv Energy Mater 2:1477–1482. https://doi.org/10.1002/aenm.201200304
Bensafi T, Hadji D, Yahiaoui A, Argoub K, Hachemaoui A, Kenane A, Baroudi B, Toubal K, Djafri A, Benkouider AM (2021) Synthesis, characterization and DFT calculations of linear and NLO properties of novel (Z)-5-benzylidene-3-N(4-methylphenyl)-2-thioxothiazolidin-4-one. J Sulfur Chem 42:645–663. https://doi.org/10.1080/17415993.2021.1951729
Benmohammed A, Hadji D, Guendouzi A, Mouchaal Y, Djafri A, Khelil A (2021) Synthesis, characterization, linear and NLO properties of novel N-(2,4-dinitrobenzylidene)-3-Chlorobenzenamine Schiff Base: combined experimental and DFT calculations. J Electron Mater 50:5282–5293. https://doi.org/10.1007/s11664-021-09046-9
Hadji D (2021) Phosphates branching effect on the structure, linear and NLO properties of linear phosphazenes. Mater Chem Phys 262:124280. https://doi.org/10.1016/j.matchemphys.2021.124280
Boukabene M, Brahim H, Hadji D, Guendouzi A (2020) Theoretical study of geometric, optical, nonlinear optical, UV–Vis spectra and phosphorescence properties of iridium(III) complexes based on 5-nitro-2-(2′,4′-difluorophenyl)pyridyl. Theor Chem Acc 139:47. https://doi.org/10.1007/s00214-020-2560-9
Kenane A, Hadji D, Argoub K, Yahiaoui A, Hachemaoui A, Toubal K, Benkouider AM, Rasoga O, Stanculescu A, Galca A (2023) Efficient NLO materials based on poly(ortho-anisidine) and polyaniline: a quantum chemical study. J Electron Mater 52:530–539. https://doi.org/10.1007/s11664-022-10022-0
Hadji D, Rahmouni A, Hammoutène D, Zekri O (2019) First theoretical study of linear and nonlinear optical properties of diphenyl ferrocenyl butene derivatives. J Mol Liq 286:110939. https://doi.org/10.1016/j.molliq.2019.110939
Haroon M, Janjua MRSA (2022) Exploring the effect of end-capped modifications of carbazole-based fullerene-free acceptor molecules for high-performance indoor organic solar cell applications. J Comput Electron 21:40–51. https://doi.org/10.1007/s10825-021-01838-w
Irfan A, Hussien M, Mehboob MY, Aziz A, Janjua MRSA (2022) Learning from fullerenes and predicting for Y6: machine learning and high-throughput screening of small molecule donors for organic solar cells. Energy Technol 10:2101096. https://doi.org/10.1002/ente.202101096
Mahmood R, Janjua MRSA, Jamil S (2017) DFT molecular simulation for design and effect of core bridging acceptors (BA) on NLO response: first theoretical framework to enhance nonlinearity through BA. J Clust Sci 28:3175–3183. https://doi.org/10.1007/s10876-017-1287-9
Janjua MRSA (2017) First theoretical framework of di-substituted donor moieties of triphenylamine and carbazole for NLO properties: quantum paradigms of interactive molecular computation. Mol Simul 43:1539–1545. https://doi.org/10.1080/08927022.2017.1332413
Merouane A, Mostefai A, Hadji D, Rahmouni A, Bouchekara M, Ramdani A, Taleb S (2020) Theoretical insights into the static chemical reactivity and NLO properties of some conjugated carbonyl compounds: case of 5-aminopenta-2,4-dienal derivatives. Monatshefte Für Chemie - Chem Mon 151:1095–1109. https://doi.org/10.1007/s00706-020-02653-y
Hadji D, Brahim H (2018) Structural, optical and nonlinear optical properties and TD-DFT analysis of heteroleptic bis-cyclometalated iridium(III) complex containing 2-phenylpyridine and picolinate ligands. Theor Chem Acc 137:180. https://doi.org/10.1007/s00214-018-2396-8
Gheribi R, Hadji D, Ghallab R, Medjani M, Benslimane M, Trifa C, Dénès G, Merazig H (2022) Synthesis, spectroscopic characterization, crystal structure, Hirshfeld surface analysis, linear and NLO properties of new hybrid compound based on tin fluoride oxalate and organic amine molecule (C12N2H9)2[SnF2(C2O4)2]2H2O. J Mol Struct 1248:131392. https://doi.org/10.1016/j.molstruc.2021.131392
Baroudi B, Argoub K, Hadji D, Benkouider AM, Toubal K, Yahiaoui A, Djafri A (2020) Synthesis and DFT calculations of linear and nonlinear optical responses of novel 2-thioxo-3-N, (4-methylphenyl) thiazolidine-4 one. J Sulfur Chem 41:310–325. https://doi.org/10.1080/17415993.2020.1736073
Basharat M, Hadji D (2022) Theoretical insights into the nonlinear optical properties of cyclotriphosphazene (P3N3Cl6), tris(4–hydroxyphenyl) ethane and their various inorganic–organic hybrid derivatives. J Mater Sci 57:6971–6987. https://doi.org/10.1007/s10853-022-07088-w
Hadji D, Benmohammed A, Mouchaal Y, Djafri A (2023) Synthesis and characterization of novel thiosemicarbazide for nonlinear optical applications: combined experimental and theoretical study. Rev Roum Chim 68:463–471. https://doi.org/10.33224/rrch.2023.68.9.07
Bekki Y, Hadji D, Guendouzi A, Houari B, Elkeurti M (2022) Linear and nonlinear optical properties of anhydride derivatives: a theoretical investigation. Chem Data Collect 37:100809. https://doi.org/10.1016/j.cdc.2021.100809
Dhaef HK, Al-Asadi RH, Shenta AA, Mohammed MK (2021) Novel bis maleimide derivatives containing azo group: synthesis, corrosion inhibition, and theoretical study. Indones J Chem 21:1212. https://doi.org/10.22146/ijc.64614
Hadji D, Rahmouni A (2015) Theoretical study of nonlinear optical properties of some azoic dyes. Mediterr J Chem 4:185–192. https://doi.org/10.13171/mjc.4.4.2015.15.07.22.50/hadji
Hadji D, Haddad B, Brandán SA, Panja SK, Paolone A, Drai M, Villemin D, Bresson S, Rahmouni M (2020) Synthesis, NMR, Raman, thermal and nonlinear optical properties of dicationic ionic liquids from experimental and theoretical studies. J Mol Struct 1220:128713. https://doi.org/10.1016/j.molstruc.2020.128713
Hadji D, Champagne B (2019) First principles investigation of the polarizability and first hyperpolarizability of anhydride derivatives. Chem Africa 2:443. https://doi.org/10.1007/s42250-019-00060-3
Hadji D, Bensafi T (2024) Deeper insights on the nonlinear optical properties of O-acylated pyrazoles. J Electron Mater 53:1868–1883. https://doi.org/10.1007/s11664-024-10954-9
Adamo C, Cossi M, Scalmani G, Barone V (1999) Accurate static polarizabilities by density functional theory: assessment of the PBE0 model. Chem Phys Lett 307:265–271. https://doi.org/10.1016/S0009-2614(99)00515-1
Bensafi T, Hadji D (2024) Quantum chemical exploration of linear and nonlinear optical characteristics in C-acylated pyrazoles. Opt Quantum Electron (Accepted paper)
Cicač-Hudi M, Feil CM, Birchall N, Nieger M, Gudat D (2022) A PH-functionalized dicationic bis(imidazolio)diphosphine. Dalton Trans 51:998–1007. https://doi.org/10.1039/d1dt03978d
Ejuh GW, Fonkem C, Tadjouteu Assatse Y, Yossa Kamsi RA, Nya T, Ndukum LP, Ndjaka JMB (2020) Study of the structural, chemical descriptors and optoelectronic properties of the drugs hydroxychloroquine and azithromycin. Heliyon 6:04647. https://doi.org/10.1016/j.heliyon.2020.e04647
Cherif FY, Hadji D, Benhalima N (2023) Molecular structure, linear, and nonlinear optical properties of piperazine-1,4-diium bis 2,4,6-trinitrophenolate: a theoretical investigation. Phys Chem Res 11:33–48. https://doi.org/10.22036/pcr.2022.330752.2035
Mohsenpour Z, Shakerzadeh E, Zare M (2017) Quantum chemical description of formaldehyde (HCHO), acetaldehyde (CH3CHO) and propanal (CH3CH2CHO) pollutants adsorption behaviors onto the bowl-shaped B36 nanosheet. Adsorption 23:1041–1053. https://doi.org/10.1007/s10450-017-9913-2
Hadji D, Rahmouni A (2016) Molecular structure, linear and nonlinear optical properties of some cyclic phosphazenes: a theoretical investigation. J Mol Struct 1106:343–351. https://doi.org/10.1016/j.molstruc.2015.10.033
Nourai NEH, Sebih F, Hadji D, Allal FZ, Dib S, Kambouche N, Rolland V, Bellahouel-Benzine S (2024) Nonlinear optical and antimicrobial activity of N-acyl glycine derivatives. J Mol Liq 398:124260. https://doi.org/10.1016/j.molliq.2024.124260
Cunha S, Rodovalho W, Azevedo NR, de O M. Mendonça, Lariucci C, Vencato I (2002) The Michael reaction of enaminones with N-(p-tolyl)-maleimide: synthesis and structural analysis of succinimide-enaminones. J Braz Chem Soc 13:629–634. https://doi.org/10.1590/S0103-50532002000500014
Cojocaru C, Airinei A, Fifere N (2013) Molecular structure and modeling studies of azobenzene derivatives containing maleimide groups. Springerplus 2:586. https://doi.org/10.1186/2193-1801-2-586
Haroon M, Janjua MRSA (2021) High-throughput designing and investigation of D-A−π–A-type donor materials for potential application in greenhouse-integrated solar cells. Energy Fuels 35:12461–12472. https://doi.org/10.1021/acs.energyfuels.1c01726
Mehboob MY, Hussain R, Adnan M, Saira UF, Irshad Z, Janjua MRSA (2022) Theoretical modelling of novel indandione-based donor molecules for organic solar cell applications. J Phys Chem Solids 162:110508. https://doi.org/10.1016/j.jpcs.2021.110508
Janjua MRSA (2021) How does bridging core modification alter the photovoltaic characteristics of triphenylamine-based hole transport materials? Theoretical Understanding and Prediction. Chem - A Eur J 27:4197–4210. https://doi.org/10.1002/chem.202004299
Janjua MRSA (2022) Photovoltaic properties and enhancement in near-infrared light absorption capabilities of acceptor materials for organic solar cell applications: a quantum chemical perspective via DFT. J Phys Chem Solids 171:110996. https://doi.org/10.1016/j.jpcs.2022.110996
Janjua MRSA (2022) All-small-molecule organic solar cells with high fill factor and enhanced open-circuit voltage with 18.25% PCE: physical insights from quantum chemical calculations. Spectrochim Acta A Mol Biomol Spectrosc 279:121487. https://doi.org/10.1016/j.saa.2022.121487
Yadav SB, Taware S, Sreenath MC, Chitrambalam S, Joe IH, Sekar N (2020) Experimental and theoretical investigation of linear and nonlinear optical properties of ethyl-3-hydroxy-2-napthoate azo dyes by solvatochromic, computational aspects, and Z-scan technique. J Phys Org Chem 33:1–20. https://doi.org/10.1002/poc.4050
mohammed kadam Z, Jeber RA, Ali AH (2021) Azo dyes on the ligand(5-MeTAQ) thin films for dye sensitized solar cells applications. J Phys Conf Ser 1999:012006. https://doi.org/10.1088/1742-6596/1999/1/012006
Gester R, Torres A, Bistafa C, Araújo RS, da Silva TA, Manzoni V (2020) Theoretical study of a recently synthesized azo dyes useful for OLEDs. Mater Lett 280:128535. https://doi.org/10.1016/j.matlet.2020.128535
Shimizu T, Tanifuji N, Yoshikawa H (2022) Azo compounds as active materials of energy storage systems. Angew Chemie Int Ed 61:202206093. https://doi.org/10.1002/anie.202206093
Al-Mudhaffer MF, Al-Ahmad AY, Ali Hassan QM, Emshary CA (2016) Optical characterization and all-optical switching of benzenesulfonamide azo dye. Optik 127:1160–1166. https://doi.org/10.1016/j.ijleo.2015.08.176
Acknowledgements
The investigation was supported by the Algerian Ministry of Higher Education and Scientific Research as well as the directorate general for scientific research and technological development.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Djebar Hadji: data curation, writing—original draft preparation, visualization, investigation, software, validation, supervision.
Benamar Baroudi: conceptualization, methodology, writing—reviewing and editing.
Toufik Bensafi: conceptualization, methodology, writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethical approval
None.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper is the author’s original work, which has not been previously published elsewhere. All authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content. The paper properly credits the meaningful contributions of all the co-authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hadji, D., Baroudi, B. & Bensafi, T. Nonlinear optical properties of azo sulfonamide derivatives. J Mol Model 30, 117 (2024). https://doi.org/10.1007/s00894-024-05915-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-024-05915-2