Abstract
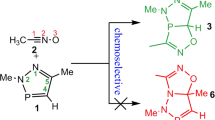
The cycloaddition reactions (32CA) of acetonitrile N-oxide and 2,5-dimethyl-2H-[1,2,3]diazarsole 1 have been examined employing the molecular electron density theory through DFT calculations at the B3LYP/6-31G ++(d,p) computational level. Investigation of the relative energies related to the competitive ortho and meta reaction paths demonstrates a high chemo-, stereo- and regioselectivity for this 32CA reaction in clear conformity with the experimental results. The topological study of the electron localisation function of the certain points of the IRC associated with the construction of the As–C and C–O single bonds shows a zwitterionic-type structure. The 32CA reaction takes place via a two-stage one-step mechanism initialised with the formation of the As–C single bond.






Similar content being viewed by others
References
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Guerin T, Molenat N, Astruc A, Pine R (2000) Arsenic speciation in some environmental samples: a comparative study of HG-GC-QFAAS and HPLC-ICP-MS methods. Appl. Organometal Chem 14:401–410
Abernathy CO, Thomasy DJ, Calderon RL (2003) Health effects and risk assessment of arsenic. The Journal of Nutrition 133:1536S–1538S
Ratnaike RN (2003) Acute and chronic arsenic toxicity. Postgrad Med J 79:391–396
Zheng Y, Tice CM, Singh SB (2014) The use of spirocyclic scaffolds in drug discovery. Bioorg Med Chem Lett 24:3673–3682
Yeung LamKo YYC, Tonnard F, Carrié R, De Sarlo F, Brandi A (1983) Cycloaddition d’oxydes de nitrile a des diazaphospholes et des composés apparentés. Réactivité comparée des doubles liaisons N = C, P = C et As = C. Tetrahedron 39:1507–1514
Domingo LR (2016) Molecular electron density theory: a modern view of reactivity in organic chemistry. Molecules 21:1319
Ríos-Gutiérrez M, Domingo LR (2019) Unravelling the Mysteries of the [3 + 2] cycloaddition reactions. Eur J Org Chem 2019:267–282
Domingo LR, Aurell MJ, Pérez P (2014) A DFT analysis of the participation of zwitterionic TACs in polar [3 + 2] cycloaddition reactions. Tetrahedron 70:4519–4525
Zeroual A, Ríos-Gutiérrez M, El Idrissi M, El Alaoui El Abdallaoui H, Domingo LR (2019) An MEDT study of the mechanism and selectivities of the [3 + 2] cycloaddition reaction of tomentosin with benzonitrile oxide. Int J Quantum Chem 1(1):1–9
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Becke AD (1993) Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J Chem Phys 96:2155–2160
Hehre WJ, Radom L, Pople Schleyer P V R J A (1986) Ab initio Molecular Orbital Theory. Wiley, New York
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 2:214–216
Schlegel H B (1994) In modern electronic structure theory. In: Yarkony DR (ed) World Scientific Publishing, Singapore
Fukui K (1970) Formulation of the reaction coordinate. J Phys Chem 74:4161–4163
González C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
González C, Schlegel HB (1991) Improved algorithms for reaction path following: higher-order implicit algorithms. J Chem Phys 95:5853–5860
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094
Cossi M, Barone V, Cammi R, Tomasi J (1996) Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett 255:327–335
Cances E, Mennucci B, Tomasi J (1997) Evaluation of solvent effects in isotropic and anisotropic dielectrics and in ionic solutions with a unified integral equation method: theoretical bases, computational implementation, and numerical applications. J Chem Phys 101:10506–10517
Barone V, Cossi M, Tomasi J (1998) Geometry optimization of molecular structures in solution by the polarizable continuum model. J Comput Chem 19:404–417
Domingo LR (2014) A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv 4:32415–32428
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Parr RG, Szentpaly LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Domingo LR, Chamorro E, Pérez P (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J Org Chem 73:4615–4624
Domingo LR, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3:1486–1494
Frisch MJ et al (2009) Gaussian 16. Gaussian Inc, Wallingford
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397–5403
Noury S, Krokidis X, Fuster F, Silvi B (1999) Computational tools for the electron localization function topological analysis. Comput Chem 23:597–604
Domingo LR, Saez JA, Zaragoza RJ, Arno M (2008) Understanding the participation of quadricyclane as nucleophile in polar [2 sigma + 2 sigma + 2 pi] cycloadditions toward electrophilic pi molecules. J Org Chem 73:8791–8799
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeroual, A., Ríos-Gutiérrez, M., El Ghozlani, M. et al. A molecular electron density theory investigation of the molecular mechanism, regioselectivity, stereoselectivity and chemoselectivity of cycloaddition reaction between acetonitrile N-oxide and 2,5-dimethyl-2H-[1,2,3]diazarsole. Theor Chem Acc 139, 37 (2020). https://doi.org/10.1007/s00214-020-2547-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-2547-6