Abstract
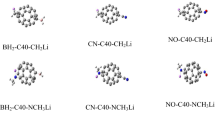
In this research, the C50 fullerene was employed as the source of the π electrons and the electron donor–acceptor groups were used to enhance its optical properties. Considerable enhancement in its electronic and optical property of C50 as the result of donor and acceptor group presence was observed. For instance, in UV–Visible absorption spectrum, the number of absorption lines significantly increase which may be the relaxation of the electronic transition selection rules. Considerably, the substituted forms of C50, have numbers of absorption bands in near infrared region. The BH2–C50-NCH3Li and NO–C50-NCH3Li molecules have superior improvement in optical properties. Finally, the donor and acceptor groups influence on non-linear optical properties (NLO) of C50 was explored and the considerable improvement in NLO properties of C50 was observed in which the NLO improvements for BH2-C50-NCH3Li and NO-C50-CH2Li cases is higher than others.






Similar content being viewed by others
References
Adamson AW, Kalyanasundaram K, Grätzel M (1993) Photosensitization and photocatalysis using inorganic and organometallic compounds. SPRINGER SCIENCE BUSINESS MEDIA, Netherlands
Zhang P, Wang M, Li C, Li X, Dong J, Sun L (2010) Chem Commun 46:8806
Pfeffer MG, Kowacs T, Wächtler M, Guthmuller J, Dietzek B, Vos JG, Rau S (2015) Angew Chem 54:6627
Ravve A (2006) Photosensitizers and Photoinitiators. In: Light-Associated Reactions of Synthetic Polymers. Springer, New York
Wu W, Duo Mao D, Xu S, Kenry Hu F, Li X, Kong D, Liu B (2018) Chem 4:1937
Whitehead K, Hedges JI (2005) J Photochem Photobiol B Bio 80:115
Koh PW, Hatta MHM, Ong ST, Yuliati L, Lee SL (2017) Photochem Photobiol B Bio 332:215
Rühle S, Shalom M, Zaban A (2010) Chem Phys Chem 11:2290
Huizhi Z, Wu L, Gao Y, Ma T (2011) J Photochem Photobiol A Chem 219:188
Yamazaki E, Murayama M, Nishikawa N, Hashimoto N, Shoyama M, Kurita O (2007) J Sol Energy 81:512
Boyo AO, Shitta MBO, Oluwa T, Adeola S (2012) Trends Appl Sci Res 7:558
Yogo T, Urano Y, Ishitsuka Y, Maniwa F, Nagano T (2005) J Am Chem Soc 127:12162
Lissi EA, Encinas MV, Lemp E, Rubio MA (1993) Chem Rev 93:699
Kou J, Dou D, Yang L (2017) Oncotarget 8:81591
Abrahamse H, Hamblin MR (2016) Biochem J 473:347
Buckingham AD (1967) Adv Chem Phys 12:107
Ekrami S, Shamlouei HR (2018) Chem Phys Lett 709:26
Atyabi SM, Shamlouei HR, Mohseni Roozbahani G, Asgari E (2020) Bull Mater Sci 43:72
Dana P, Shamlouei HR, Maleki A, Shirvan SA (2021) J Chin Chem Soc 68:959
Hamidi A, Shamlouei HR, Maleki A, Mombeini Goodajdar B (2020) J Mol Model 26:348
Harris P (2004) Philos Mag 84:3159
Curl RF, Smalley RE (1988) Science 242:1017
Vincent D, Cruickshank J (1997) Appl Opt 36:7794
Palit DK, Sapre AV, Mittal JP, Rao CNR (1992) Chem Phys Lett 195:1
Arbogast JW, Darmanyan AP, Foote CS, Diederich FN, Whetten RL, Rubin Y, Alvarez MM, Anz SJ (1991) J Phys Chem 95:11
Frisch M, Trucks G, Schlegel HB, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2009) Gaussian, Inc., Wallingford
O'boyle NM, Tenderholt AL, Langner KM (2008) J Comput Chem 29:839
Peach MJG, Helgaker T, Saiek P, Keal TW, Lutnas OB, Tozer DJ, Handy NC (2006) Phys Chem Chem Phys 8:558
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ayoubikaskooli, A., Ghaedi, A.M., Shamlouei, H.R. et al. Influence of donor–acceptor groups on the electrical and optical properties of C50 fullerene. J Mol Model 28, 7 (2022). https://doi.org/10.1007/s00894-021-05001-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-05001-x