Abstract
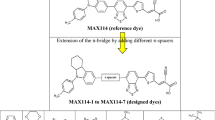
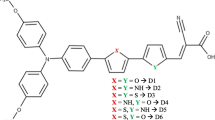
In this paper, we present a series of sensitizers to shed light on the influence of π-spacers on the performance of dye-sensitized solar cells. We have accurately calculated key properties in energy conversion, including sunlight absorption, electron injection, electron/hole reorganization energy, ionization potential (IP) and electronic affinity (EA). We chose a series of donor-π-acceptor dyes based on methyl-indole-carbazole as the electron donor group and cyano-acrylic acid as an acceptor with various π-conjugated systems. The results obtained show that, with incorporation of the thieno(3,4-b)pyrazine in the two π-spacer parts, D4 may be the best candidate among the dyes studied, due to its many advantages such as low gap energy, red-shift absorption spectra, large ΔGInj, low hole/electron reorganization energies, low IP and high EA, which indicate its better optoelectronic properties, which present more balanced transport rates and provide good injection ability.

Absorption spectra in gaz phase, obtained by CAM-B3LYP/6-31G(d,p) with solar spectrum.






Similar content being viewed by others
References
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737
Urbani M, Grätzel M, Nazeeruddin MK, Torres T (2014) Meso-substituted porphyrins for dye-sensitized solar cells. Chem Rev 114:12330–12396
Bai Y, Mora-Sero I, De Angelis F et al (2014) Titanium dioxide nanomaterials for photovoltaic applications. Chem Rev 114:10095–10130
Mathew S, Yella A, Gao P et al (2014) Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat Chem 6:242
Sauvage F, Chen D, Comte P et al (2010) Dye-sensitized solar cells employing a single film of mesoporous TiO2 beads achieve power conversion efficiencies over 10%. ACS Nano 4:4420–4425
Gao F, Wang Y, Shi D et al (2008) Enhance the optical absorptivity of nanocrystalline TiO2 film with high molar extinction coefficient ruthenium sensitizers for high performance dye-sensitized solar cells. J Am Chem Soc 130:10720–10728
Kakiage K, Aoyama Y, Yano T et al (2015) Fabrication of a high-performance dye-sensitized solar cell with 12.8% conversion efficiency using organic silyl-anchor dyes. Chem Commun 51:6315–6317
Yao Z, Zhang M, Wu H et al (2015) Donor/acceptor indenoperylene dye for highly efficient organic dye-sensitized solar cells. J Am Chem Soc 137:3799–3802
Yao Z, Zhang M, Li R et al (2015) A metal-free N-annulated Thienocyclopentaperylene dye: power conversion efficiency of 12% for dye-sensitized solar cells. Angew Chem 127:6092–6096
Kakiage K, Aoyama Y, Yano T et al (2014) An achievement of over 12 percent efficiency in an organic dye-sensitized solar cell. Chem Commun 50:6379–6381
Kim B, Chung K, Kim J (2013) Molecular design principle of all-organic dyes for dye-sensitized solar cells. Chem Eur J 19:5220–5230
Hagfeldt A, Boschloo G, Sun L et al (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Koumura N, Wang Z-S, Mori S et al (2006) Alkyl-functionalized organic dyes for efficient molecular photovoltaics. J Am Chem Soc 128:14256–14257
Lee MJ, Seo KD, Song HM et al (2011) Novel D-π-a system based on zinc-porphyrin derivatives for highly efficient dye-sensitised solar cells. Tetrahedron Lett 52:3879–3882
Liang M, Chen J (2013) Arylamine organic dyes for dye-sensitized solar cells. Chem Soc Rev 42:3453–3488
Cai L, Moehl T, Moon S-J et al (2013) 4, 9-Dihydro-4, 4, 9, 9-tetrahexyl-s-indaceno [1, 2-b: 5, 6-b′] dithiophene as a π-spacer of donor− π–acceptor dye and its photovoltaic performance with liquid and solid-state dye-sensitized solar cells. Org Lett 16:106–109
Liu J, Yang X, Islam A et al (2013) Efficient metal-free sensitizers bearing circle chain embracing π-spacers for dye-sensitized solar cells. J Mater Chem A 1:10889–10897
Liu B, Li W, Wang B et al (2013) Influence of different anchoring groups in indoline dyes for dye-sensitized solar cells: Electron injection, impedance and charge recombination. J Power Sources 234:139–146
Zhao Z, Xu X, Wang H et al (2008) Zigzag molecules from pyrene-modified carbazole oligomers: synthesis, characterization, and application in OLEDs. J Org Chem 73:594–602
Hwang S, Lee JH, Park C et al (2007) A highly efficient organic sensitizer for dye-sensitized solar cells. Chem Commun 4887–4889
Xu M, Li R, Pootrakulchote N et al (2008) Energy-level and molecular engineering of organic D-π-a sensitizers in dye-sensitized solar cells. J Phys Chem C 112:19770–19776
Zhang G, Bai Y, Li R et al (2009) Employ a bisthienothiophene linker to construct an organic chromophore for efficient and stable dye-sensitized solar cells. Energy Environ Sci 2:92–95
Chang YJ, Wu Y-J, Chou P-T et al (2014) Triarylene linked spacer effect for dye-sensitized solar cells. Thin Solid Films 558:330–336
Luo C, Bi W, Deng S et al (2014) Indolo [3, 2, 1-jk] carbazole derivatives-sensitized solar cells: effect of π-bridges on the performance of cells. J Phys Chem C 118:14211–14217
Li R, Lv X, Shi D et al (2009) Dye-sensitized solar cells based on organic sensitizers with different conjugated linkers: furan, bifuran, thiophene, bithiophene, selenophene, and biselenophene. J Phys Chem C 113:7469–7479
Seo KD, Choi IT, Kim HK (2015) Organic dyes with well-defined structures for highly efficient dye-sensitised solar cells based on a cobalt electrolyte. Chem Eur J 21:14804–14811
Srinivas K, Yesudas K, Bhanuprakash K et al (2009) A combined experimental and computational investigation of anthracene based sensitizers for DSSC: comparison of cyanoacrylic and malonic acid electron withdrawing groups binding onto the TiO2 anatase (101) surface. J Phys Chem C 113:20117–20126
Narayan MR (2012) Dye sensitized solar cells based on natural photosensitizers. Renew Sust Energ Rev 16:208–215
Zhang J, Li H-B, Sun S-L et al (2012) Density functional theory characterization and design of high-performance diarylamine-fluorene dyes with different π spacers for dye-sensitized solar cells. J Mater Chem 22:568–576
Feng J, Jiao Y, Ma W et al (2013) First principles design of dye molecules with ullazine donor for dye sensitized solar cells. J Phys Chem C 117:3772–3778
Preat J, Jacquemin D, Michaux C, Perpète EA (2010) Improvement of the efficiency of thiophene-bridged compounds for dye-sensitized solar cells. Chem Phys 376:56–68
Mahmood A, Tahir MH, Irfan A et al (2015) Heterocyclic azo dyes for dye sensitized solar cells: a quantum chemical study. Comput Theor Chem 1066:94–99
Ding W-L, Wang D-M, Geng Z-Y et al (2013) Density functional theory characterization and verification of high-performance indoline dyes with D–A–π–A architecture for dye-sensitized solar cells. Dyes Pigments 98:125–135
Sang-Aroon W, Saekow S, Amornkitbamrung V (2012) Density functional theory study on the electronic structure of Monascus dyes as photosensitizer for dye-sensitized solar cells. J Photochem Photobiol A Chem 236:35–40. https://doi.org/10.1016/j.jphotochem.2012.03.014
MJ Frisch, GW Trucks, HB Schlegel et al (2009) Gaussian09. Gaussian. Inc, Wallingford CT
Preat J, Michaux C, Jacquemin D, Perpete EA (2009) Enhanced efficiency of organic dye-sensitized solar cells: triphenylamine derivatives. J Phys Chem C 113:16821–16833
Fahim ZME, Bouzzine SM, Youssef AA, et al (2018) Ground state geometries, UV/Vis absorption spectra and charge transfer properties of Triphenylamine-Thiophenes based dyes for DSSCs: a TD-DFT benchmark study. Comput Theor Chem 1125:39–48
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Becke AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98:1372–1377
Casida ME, Jamorski C, Casida KC, Salahub DR (1998) Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: characterization and correction of the time-dependent local density approximation ionization threshold. J Chem Phys 108:4439–4449
Wong MW, Frisch MJ, Wiberg KB (1991) Solvent effects. 1. The mediation of electrostatic effects by solvents. J Am Chem Soc 113:4776–4782
Mohammadi N, Wang F (2014) First-principles study of Carbz-PAHTDDT dye sensitizer and two Carbz-derived dyes for dye sensitized solar cells. J Mol Model 20:2177
Fan W, Deng W (2013) Incorporation of thiadiazole derivatives as π-spacer to construct efficient metal-free organic dye sensitizers for dye-sensitized solar cells: a theoretical study. Commun Comput Chem 1:152–170
Mo Y, Lin Z, Wu W, Zhang Q (1996) Bond-distorted orbitals and effects of hybridization and resonance on C− C bond lengths. J Phys Chem 100:11569–11572
Kręglewski M (1989) The geometry and inversion-internal rotation potential function of methylamine. J Mol Spectrosc 133:10–21
Pearson Jr R, Lovas FJ (1977) Microwave spectrum and molecular structure of methylenimine (CH2NH). J Chem Phys 66:4149–4156
Zhang Z-L, Zou L-Y, Ren A-M et al (2013) Theoretical studies on the electronic structures and optical properties of star-shaped triazatruxene/heterofluorene co-polymers. Dyes Pigments 96:349–363
Ait Aicha Y, Bouzzine SM, Fahim ZM et al (2014) Quantum chemical investigations study of the effect of electron donor units on the structural, electronic and optoelectronic properties of diarylthienopyrazine analogs. Comput Theor Chem 1036. https://doi.org/10.1016/j.comptc.2014.03.008
Han D, Zhang G, Cai H et al (2013) Theoretical studies on the electronic structures and spectral properties of a series of bis-cyclometalated iridium (III) complexes using density functional theory. J Lumin 138:223–228
Fahim ZME, Bouzzine SM, Aicha YA et al (2018) The bridged effect on the geometric, optoelectronic and charge transfer properties of the triphenylamine–bithiophene-based dyes: a DFT study. Res Chem Intermed 44:2009–2023
Acknowledgments
The authors thank the Volubilis Program (N° Ma/11/248) for the purchase of Gaussian09. One of the authors (İ.S.) greatly thanks Bitlis Eren University for supporting this study by Gaussian 09 W and GaussView5.0 softwares.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Mzioui, S., Bouzzine, S.M., Sidir, İ. et al. Theoretical investigation on π-spacer effect of the D–π–A organic dyes for dye-sensitized solar cell applications: a DFT and TD-BHandH study. J Mol Model 25, 92 (2019). https://doi.org/10.1007/s00894-019-3963-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-3963-1