Abstract
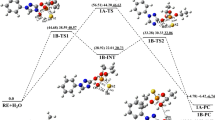
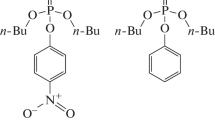
In this work the neutral or spontaneous hydrolysis of paraoxon, one of the most popular organophosphate pesticides, in aqueous solution was investigated at the DFT and MP2 levels of theory, using a combination of local solvation of the phosphoryl group with explicit water molecules, and treating the long range solvent effects using continuum solvation model. In contrast to the alkaline hydrolysis, the neutral hydrolysis takes place in two steps, through an AN + DN mechanism, with formation of a pentacoordinate phosphorane intermediate. The reaction has activation free energies of 31.8 and 1.9 kcal mol−1 for the first and second steps, respectively, and has an overall reaction free energy of −9.3 kcal mol−1, computed at the MP2/6–311++G(2d,2p)//B3LYP/6–31+G(d) level of theory. The reaction proceeds through a sequence of proton transfer processes from the attacking water molecule and ends with the protonation of the nitrophenolate leaving group. Explicit description of the local solvating water molecules is essential to describe the proton transfer processes along the reaction coordinate and to stabilize the pentacoordinate intermediate formed. The neutral hydrolysis is very slow and has an overall rate constant of 3.05 × 10−11 s−1, computed at the MP2/6–311++G(2d,2p)//B3LYP/6–31+G(d) level of theory. This result, in conjunction with the sensitivity of the rate constant to the experimental conditions, indicates that the hydrolysis of paraoxon in aqueous solution can be even slower than predicted experimentally.



Similar content being viewed by others
References
Knowles JR (1980) Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem 49:877–919
Westheimer FH (1987) Why nature chose phosphates. Science 235:1173–1178
Cleland WW, Hengge AC (2006) Enzymatic mechanisms of phosphate and sulfate transfer. Chem Rev 106:3252–3278
Lassila JK, Zalatam JG, Herschlag D (2011) Biological phosphoryl-transfer reactions: understanding mechanism and catalysis. Annu Rev Biochem 80:669–702
Karmelin SCL, Sharma PK, Prasad RB, Warshel A (2013) Why nature really chose phosphate. Q Rev Biophys 46:1–132
Kirby AJ, Nome F (2015) Fundamentals of phosphate transfer. Acc Chem Res 48:1806–1814
Prasad BR, Plotnikov NV, Warshel A (2013) Addressing open questions about phosphate hydrolysis pathway by careful free energy mapping. J Phys Chem B 117:153–163
Plotnikov NV, Prasad BR, Chakrabarty S, Chu ZT, Warshel A (2013) Quantifying the mechanism of phosphate monoester hydrolysis in aqueous solution by evaluating the relevant ab initio QM/MM free energy surfaces. J Phys Chem B 117:12807–12819
Kirby AJ, Mora JR, Nome F (1834) New light on phosphate transfer from triesters. Biochim Biophys Acta 2013:454–463
Kirby AJ, Souza BS, Nome F (2015) Structure and reactivity of phosphate diesters. Dependence of the nonleaving group. Can J Chem 93:422–427
Duarte F, Barrozo A, Aqvist J, Williams NH, Karmelin SCL (2016) The competing mechanisms of phosphate monoester dianion hydrolysis. J Am Chem Soc 138:10664–10673
Ferreira DEC, Boldt IS, De Almeida WB, Rocha WR, Nome F (2014) Quantum mechanical/effective fragment potential (QM/EFP) study of phosphate diester cleavage in aqueous solution. Comput Theor Chem 1043:5–12
Pereira ES, Da Silva JCS, Brandão TAS, Rocha WR (2016) Phosphorane lifetime and stereo-electronic effects along the alkaline hydrolysis of phosphate esters. Phys Chem Chem Phys 18:18255–18267
Lad C, Williams NH, Wolfenden R (2003) The rate of hydrolysis of phosphomonoester dianions and the exceptional catalytic proficiencies of protein and inositol phosphatases. Proc Natl Acad Sci 100:5607–5610
Schroeder GK, Lad C, Wyman P, Williams NH, Wolfenden R (2006) The time required for water attack at the phosphorus atom of simple phosphodiesters and of DNA. Proc Natl Acad Sci 103:4052–4055
Aubert AD, Li Y, Raushel FM (2004) Mechanism for the hydrolysis of organophosphates by the bacterial phosphotriesterase. Biochemistry 43:5707–5715
Mitic N, Smith SJ, Neves A, Guddat LW, Gahan LR, Schenk G (2006) The catalytic mechanisms of binuclear Metallohydrolases. Chem Rev 106:3338–3363
Erickson BE (2017) Chlorpyrifos Sparks outcry. Centaurus 95:27–30
Kennedy DJ, Mayer BP, Baker SE, Valdez CA (2015) Kinetics and speciation of paraoxon hydrolysis by zinc (II)-azamacrocyclic catalysts. Inorg Chim Acta 436:123–131
Benschop HP, De Jong LPA (1988) Nerve agent stereoisomers: analysis, isolation, and toxicology. Acc Chem Res 21:368–374
Kirby AJ, Holfelder F (2009) From enzyme models to model enzymes. Royal Society of Chemistry, Cambridge
Bigley AN, Raushel FM (1834) Catalytic mechanisms for phosphotriesterases. Biochim Biophys Acta 2013:443–453
Dumas DP, Caldwell SR, Wild JR, Raushel FM (1989) Purification and properties of the phosphotriesterase from Pseudomonas diminuta. J Biol Chem 264:19659–19665
Purcell J, Hengge AC (2005) The thermodynamics of phosphate versus Phosphorothioate Ester hydrolysis. J Organomet Chem 70:8437–8442
Caldwell SR, Raushel FM, Weiss PM, Cleland WW (1991) Transition-state structures for enzymatic and alkaline Phosphotriester hydrolysis. Biochemistry 30:7444–7450
Zheng F, Zhan CG, Ornstein RL (2001) Theoretical studies of reaction pathways and energy barriers for alkaline of phosphotriesterase substrates and related toxic phosphofluoridate nerve agents. J Chem Soc Perkin Trans 2:2355–2363
Khan MAS, Bandyopadhyay T, Ganguly B (2012) Probing the simulant behavior of PNPDPP toward parathion and paraoxon: a computational study. J Mol Graph Model 34:10–17
Canut VL, Pernía JJRP, Castillo R, Moliner V, Tuñón I (2012) Hydrolysis of phosphotriesters: a theoretical analysis of the enzymatic and solution mechanisms. Chem Eur J 18:9612–9621
Tarrat N (2010) Alkaline hydrolysis of phosphate triesters in solution: stepwise or concerted? A theoretical study. J Mol Struct THEOCHEM 941:56–60
Zhang L, Xie D, Xu D, Guo H (2007) Supermolecule density functional calculations suggest a key role for solvent in alkaline hydrolysis of p-Nitrophenyl phosphate. Chem Commun 0:1638–1640
Mora JR, Kirby AJ, Nome F (2012) Theoretical study of the importance of the spectator groups on the hydrolysis of phosphate Triesters. J Organomet Chem 77:7061–7070
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Szabo A, Ostlund NS (1996) Modern quantum chemistry: introduction to advanced electronic structure theory. Dover, New York
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular orbital methods. 9. Extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54(1971):724
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28:213–222
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241
Fukui K (1981) The path of chemical reactions - the IRC approach. Acc Chem Res 14:363–368
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154–2161
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B:113.6378–113.6396
Breneman CM, Wiberg KB (1990) Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in Formamide conformational analysis. J Comput Chem 11:361–373
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision E.01. Gaussian Inc, Wallingford
Xia F, Tian K, Zhu H (2013) Density functional calculations on alcoholysis and thiolysis of phosphate triesters: stepwise or concerted? Comput Theor Chem 1017:60–71
Gomaa HM, Faust SD (1972) Chemical hydrolysis and oxidation of parathion and paraoxon in aquatic environments. In: Faust SD (ed) Advances in chemistry: fate of organic pesticides in aquatic environment, chap 10, pp 189–209. American Chemical Society, Washington, DC
Wanamaker EC, Chingas GC, Mcdougal OM (2013) Parathion hydrolysis revisited: in situ aqueous kinectics by 1H NMR. Environ Sci Technol 47:9267–9273
Weber K (1976) Degradation of parathion in seawater. Water Res. 10:237–241
Ketelaar JAA (1950) Some p-nitrophenyl derivatives of o-phosphoricand o-thiophosphoric acid. Recl Trav Chim Pays-Bas 69:649–658
Cowart RP, Bonner FL, Epps EA (1971) Rate of hydrolysis of seven organophosphate pesticides. Bull Environ Contam Toxicol 6:231–234
Chagas MA, Pereira ES, Godinho MPB, Da Silva JCS, Rocha WR (2018) A base mechanism to the hydrolysis of phosphate triester promoted by the Cd2+/Cd2+ active site of phosphotriesterase: a computational study. Inorg Chem 57:5888–5902
Acknowledgments
The authors would like to thank the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, INCT-Catálise) and FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais) for the financial support and research grants.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection XIX - Brazilian Symposium of Theoretical Chemistry (SBQT2017)
Electronic supplementary material
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Chagas, M.A., Pereira, E.S., Da Silva, J.C.S. et al. Theoretical investigation of the neutral hydrolysis of diethyl 4-nitrophenyl phosphate (paraoxon) in aqueous solution. J Mol Model 24, 259 (2018). https://doi.org/10.1007/s00894-018-3798-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3798-1