Abstract
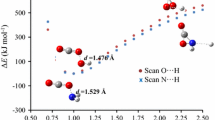
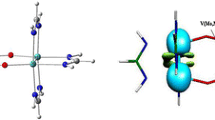
In this computational study the interaction of NO., NO+, and NO− with H2O: [NO--H2O]., 1 ., [NO--H2O]+, 1 +, and [NO--H2O]−, 1 − was analysed. The optimized geometries indicate that the relative position of NO and H2O depends on the total charge: (ON.--H-OH), (NO−--H-OH), and (ON+--OH2). Moreover, atomic spin density along with frontier molecular orbitals help to identify the preferred reduction or oxidation sites on the nitric oxide. Thus, quantum theory of atoms in molecules (QTAIM), electron localization function (ELF), and natural bond-bond polarizability (NBBP) methods aid to quantify the electron delocalization level between NO and H2O, 1 + > 1 . > 1 −, and show the predominantly ionic, and covalent character to inter-molecular, and intra-molecular chemical bonds, respectively. Furthermore, the natural bond orbital (NBO) and localized molecular orbital energy decomposition analysis (LMO-EDA) methods enable energy analyses of the interaction between NO and H2O in the complexes 1 ., 1 +, and 1 −. Where, the first method showed that the interaction between the natural bond orbitals in 1 − is more favorable, than in 1 +, and less in 1 ., however, the second method designates that the total interaction energy is lower for 1 + in relation to 1 − and 1 ., due mainly to the electrostatic component. As a final point, analysis of the electrostatic potential surfaces provides a clear and direct explanation for the relative position of the monomers. It also shows that the predominant Coulombic attraction between H2O and the charged NO+, and NO− compounds will be stronger in relation to the neutral NO..

ᅟ








Similar content being viewed by others
References
Desai SR, Feigerle CS, Miller JC (1994) Laser ionization mass-spectrometry of homogeneous and binary molecular clusters of nitric-oxide. J Chem Phys 101:4526–4535
Finlayson-Pitts BJ, Pitts JN (1986) Atmospheric chemistry: fundamentals and experimental techniques. Wiley, Chichester
Snyder SH, Bredt DS (1992) Biological roles of nitric oxide. Sci Am 266:68–71
Cybulski H, Zuchowski PS, Fernandez B, Sadlej J (2009) The water-nitric oxide intermolecular potential-energy surface revisited. J Chem Phys 130:104303
Hughes MN (1999) Relationships between nitric oxide, nitroxyl ion, nitrosonium cation and peroxynitrite. Biochim Biophys Acta 1411:263–272
Roszak S (1994) A theoretical study of NO(−) complexes with neutral water solvent molecules. Chem Phys Lett 221:255–258
Atkins P, de Paula J (2006) Physical chemistry. New York, Oxford
Lommerse JPM, Stone AJ, Taylor R, Allen FH (1996) The nature and geometry of intermolecular interactions between halogens and oxygen or nitrogen. J Am Chem Soc 118:3108–3116
Bissantz C, Kuhn B, Stahl M (2010) A medicinal chemist’s guide to molecular interactions. J Med Chem 53:5061–5084
Myszkiewicz G, Sadlej J (2000) Ab initio study for the intermolecular potential of the water-nitric oxide complex. Chem Phys Lett 318:232–239
Hammam E, Lee EPF, Dyke JM (2000) Ab initio molecular orbital calculations on NO+ center dot (H20)(n) cluster ions. Part I: minimum-energy structures and possible routes to nitrous acid formation. J Phys Chem A 104:4571–4580
Myshakin EM, Jordan KD, Robertson WH, Weddle GH, Johnson MA (2003) Dominant structural motifs of NO-center dot(H2O)(n) complexes: infrared spectroscopic and ab initio studies. J Chem Phys 118:4945–4953
Zheng WX, Wang WZ, Pu XM, Tian AM, Wong NB (2003) Effect of CP-corrected gradient optimization on the water-radical (anion) dimer hypersurface. J Mol Struc-Theochem 631:171–180
Dozova N, Krim L, Alikhani ME, Lacome N (2006) Vibrational spectra and structures of H2O-NO, HDO-NO, and D2O-NO complexes. An IR matrix isolation and DFT study. J Phys Chem A 110:11617–11626
Zhou ZW, Todd BD, Travis KP, Sadus RJ (2005) A molecular dynamics study of nitric oxide in water: diffusion and structure. J Chem Phys 123:054505
Mack P, Dyke JM, Wright TG (1997) Calculated thermodynamics of reactions involving NO+.X complexes (where X=H2O, N2 and CO2). Chem Phys 218:243–256
Orenha RP, Galembeck SE (2014) Molecular orbitals of NO, NO+, and NO-: a computational quantum chemistry experiment. J Chem Educ 91:1064–1069
Schaftenaar G, Noordik JH (2000) Molden: a pre- and post-processing program for molecular and electronic structures. J Comput Aided Mol Design 14:123–134
Becke AD (1993) Density-functional thermochemistry.III. The role of exact exchange. J Chem Phys 98:5648–5652
Harihara PC, Pople JA (1973) Influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28:213–222
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE Jr, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, revision D.01. Gaussian Inc, Wallingford
Bader RFW (1998) A bond path: a universal indicator of bonded interactions. J Phys Chem A 102:7314–7323
Todd A, Keith TK (2016) AIMAll (version 14.11.23). Gristmill Software, Overland Park. aim.tkgristmill.com
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Frenking G, Shaik S (2014) The chemical bond: fundamental aspects of chemical bonding. Wiley-VCH, Weinheim
Zimmerman HE, Weinhold F (2013) Natural bond-bond polarizability: a Huckel-like electronic delocalization index. J Org Chem 78:1844–1850
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Landis CR, Weinhold F (2013) NBO 6.0. Theoretical Chemistry Institute, University of Wisconsin, Madison
Su P, Li H (2009) Energy decomposition analysis of covalent bonds and intermolecular interactions. J Chem Phys 131:074109
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Jmol: an open-source Java viewer for chemical structures in 3D. http://jmol.org/. Accessed 2 Feb 2016
Natural Bond Orbitals (NBO) in Organic Chemistry. http://chemgplus.blogspot.com.br/2013/08/jmol-nbo-visualization-helper.html. Accessed 2 Feb 2016
Dennington R, Keith T, Millam J (2009) GaussView 5.0. Semichem Inc, Shawnee Mission
Marvin 5.12.3, 2013, ChemAxon. http://www.chemaxon.com. Accessed 2 Feb 2016
Eskandari K, Van Alsenoy C (2014) Hydrogen-hydrogen interaction in planar biphenyl: a theoretical study based on the interacting quantum atoms and Hirshfeld atomic energy partitioning methods. J Comput Chem 35:1883–1889
Rauk A (1994) Orbital interactions of organic chemistry. Wiley, New York
IUPAC Compendium of Chemical Terminology—the Gold book. http://goldbook.iupac.org/. Accessed 2 Feb 2016
Baranac-Stojanovic M, Aleksic J, Stojanovic M (2015) Energy decomposition analysis of gauche preference in 2-haloethanol, 2-haloethylamine (halogen = F, Cl), their protonated forms and anti preference in 1-chloro-2-fluoroethane. RSC Adv 5:22980–22995
Chen Y, Li H (2010) Intermolecular interaction in water hexamer. J Phys Chem A 114:11719–11724
Liu L, Meng L, Zhang X, Zeng Y (2016) Comparison of the directionality of the halogen, hydrogen, and lithium bonds between HOOOH and XF (X = Cl, Br, H, Li). J Mol Model 22:52
Ford KA (2013) Role of electrostatic potential in the in silico prediction of molecular bioactivation and mutagenesis. Mol Pharm 10:1171–1182
Politzer P, Lane P, Murray JS (2016) Electrostatic potentials, intralattice attractive forces and crystal densities of nitrogen-rich C, H, N, O salts. Crystals 6:7
Politzer P, Murray JS, Clark T (2015) Mathematical modeling and physical reality in noncovalent interactions. J Mol Model 21:52
Acknowledgments
The authors thank the Brazilian agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Programa de Apoio à Pós-Graduação (PROAP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant 304447/2010-2), and São Paulo Research Foundation (FAPESP, Fundação de Amparo à Pesquisa do Estado de São Paulo) (Grants 2008/02677-0 and 2014/50265-3) for financial support. S.E.G. thanks CNPq for a research fellowship (Grant 304393/2013-4). R.P.O. thanks FAPESP for graduate fellowships (grants 2011/20351-7 and 2015/15176-2). We also acknowledge Ali Faez Taha for technical assistance and Cynthia M. C. Prado Manso for revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orenha, R.P., San Gregorio, L.R. & Galembeck, S.E. Computational study of the interaction between NO, NO+, and NO− with H2O. J Mol Model 22, 276 (2016). https://doi.org/10.1007/s00894-016-3148-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3148-0