Abstract
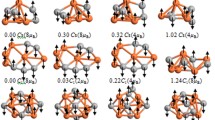
Density functional theory (DFT) has been applied to investigate the structural and electronic properties of an [(Al2O3)4]+ cluster. Since there is no structural data available from experiment, the geometry of the cluster was obtained based on a model which produced the best agreement with vibrational IR-MPD data. A range of different exchange-correlation functionals were tested, and it was concluded that the best spectral agreement was produced using the CAM-B3LYP and B3LYP functionals, respectively. To further characterize the properties of the cluster, natural bond order analysis was performed, and it was concluded that an appropriate description for the system is [Al8O12]+. The frontier orbitals and spin densities of both cation and neutral systems were considered, and it was concluded that the unrestricted singlet and triplet spin densities of the neutral [Al8O12] system were nearly degenerate, representing a di-radical, with the triplet state being lower in energy.







Similar content being viewed by others

References
Rahane AB, Deshpande MD, Kumar V (2011) Structural and electronic properties of (Al2O3)n clusters with n = 1–10 from first principles calculations. J Phys Chem C 115:1811–1821
Archibong EF, St-Amant A (1999) On the structure of Al2O3 and photoelectron spectra of Al2O2 − and Al2O3 −. J Phys Chem A 103:1109–1114
Gianotto AK, Rawlinson JW, Cossel KC, Olson JE, Appelhans AD, Groenewold GS (2004) Hydration of alumina cluster anions in the gas phase. J Am Chem Soc 126:8275–8283
Patzer ABC, Chang C, Sedlmayr E, Sϋlze D (2005) A density functional study of small AlxOy (x, y = 1–4) clusters and their thermodynamic properties. Eur Phys J D 32:329–337
Mitin AV (2011) Accurate theoretical IR and Raman spectrum of Al2O2 and Al2O3 molecules. Struct Chem 22:411–418
Shirai T, Watanabe H, Fuji M, Takahashi M (2009) Structural properties and surface characteristics on aluminum oxide powders. Ann Rep Ceram Res Lab Nagoya Inst Technol 9:23–31
Levin I, Brandon D (1998) Metastable alumina polymorphs: crystal structures and transition sequences. J Am Ceram Soc 81:1995–2012
Sierka M, Dӧbler J, Sauer J, Santambrogio G, Brümmer M, Wӧste L, Janssens E, Meijer G, Asmis KR (2007) Unexpected structures of aluminum oxide clusters in the gas phase. Agnew Chem Int Ed 46:3372–3375
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam MJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian Inc, Wallingford
Molekel version 5.4, www.cscs.ch/molekel
Chemcraft, http://www.chemcraftprog.com
Pulay P, Fogarasi G, Pongor G, Boggs JE, Vargha A (1983) Combination of theoretical ab initio and experimental information to obtain reliable harmonic force constants. Scaled quantum mechanical (QM) force fields for glyoxal, acrolein, butadiene, formaldehyde, and ethylene. J Am Chem Soc 105:7037–7047
Acknowledgments
We would like to acknowledge support from the Polish Ministry of Science and Higher Education within the statutory research number S-12/2015. Visiting professorship of Pawel M. Kozlowski at the Medical University of Gdansk was partially supported by the KNOW program. In addition, we would like to acknowledge the Cardinal Research Cluster (CRC) at the University of Louisville for ensuring computational resources.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Jaroszynska-Wolinska, J., Garabato, B.D., Alam, J. et al. Structural and electronic properties of an [(Al2O3)4]+ cluster. J Mol Model 21, 170 (2015). https://doi.org/10.1007/s00894-015-2711-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2711-4