Abstract
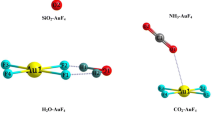
The binding behavior of coinage metal anions with some electron-deficient arenes has been investigated by MP2 calculations, and the character of interactions in these complexes has been examined by NBO analysis. The results indicate that coinage metal anions can interact with electron-deficient arenes to form anion-π, strong σ-type and hydrogen-bonding complexes. The σ-type structure is the global minimum for triazine, trifluorotriazine, hexafluorobenzene and tricyanobenzene, and the hydrogen-bonding structure is the global minimum for trifluorobenzene. There exist some differences in the stability of anion-π complexes for coinage metal anions: the anion-π complexes of Au- are minima expect for triazine complex; the anion-π complexes of Ag- are minima expect for tricyanobenzene complex; and the anion-π complexes of Cu- are not minima expect for trifluorobenzene complex. The binding strength of anion-π and hydrogen-bonding complexes for Au- is larger than that for Ag- and Cu-, but the binding strength of σ complex displays a different sequence: Cu- > Au- > Ag-. The binding behavior of coinage metal anions is more similar to that of F- than that of Cl- and Br-. The relaxed potential energy surface scans for some selected systems have been performed to help understand the interactions between coinage metal anions with electron-deficient arenes.






Similar content being viewed by others
References
Quiñonero D, Garau C, Rotger C, Frontera A, Ballester P, Costa A, Deyà PM (2002) Anion-π interactions: do they exist? Angew Chem Int Ed 41:3389–3392
Schottel BL, Chifotides HT, Dunbar KR (2008) Anion-π interactions. Chem Soc Rev 37:68–83
Mascal M, Armstrong A, Bartberger MD (2002) Anion-aromatic bonding: a case for anion recognition by π-acidic rings. J Am Chem Soc 124:6274–6276
Alkorta I, Rozas I, Elguero J (2002) Interaction of anions with perfluoro aromatic compounds. J Am Chem Soc 124:8593–8598
Frontera A (2013) Encapsulation of anions: macrocyclic receptors based on metal coordination and anion–π interactions. Coord Chem Rev 257:1716–1727
Frontera A, Quiñonero D, Deyà PM (2011) Cation–π and anion–π interactions. WIREs Comput Mol Sci 1:440–459
Frontera A, Gamez P, Mascal M, Mooibroek TJ, Reedijk J (2011) Putting anion-π interactions into perspective. Angew Chem Int Ed 50:9564–9583
Estarellas C, Bauza A, Frontera A, Quiñonero D, Deyà PM (2011) On the directionality of anion-π interactions. Phys Chem Chem Phys 13:5696–5702
Garau C, Frontera A, Quiñonero D, Ballester P, Costa A, Deyà PM (2004) Cation−π versus anion−π interactions: energetic, charge transfer, and aromatic aspects. J Phys Chem A 108:9423–9427
Garau C, Frontera A, Quiñonero D, Ballester P, Costa A, Deyà PM (2005) Approximate additivity of anion − π interactions: an Ab initio study on anion−π, anion−π2 and anion−π3 complexes. J Phys Chem A 109:9341–9345
Schneider H, Vogelhuber KM, Schinle F, Weber JM (2007) Aromatic molecules in anion recognition: electrostatics versus HBonding. J Am Chem Soc 129:13022–13026
Garau C, Frontera A, Quiñonero D, Ballester P, Costa A, Deya PM (2003) A topological analysis of the electron density in anion-π interactions. ChemPhysChem 4:1344–1348
Kim D, Lee EC, Kim KS, Tarakeshwar P (2007) Cation-π-anion interaction: a theoretical investigation of the role of induction energies. J Phys Chem A 111:7980–7986
Kim DY, Singh NJ, Kim KS (2008) Cyameluric acid as anion-π type receptor for ClO4 - and NO3 -: π-stacked and edge-to-face structures. J Chem Theory Comput 4:1401–1407
Kim DY, Singh NJ, Lee JW, Kim KS (2008) Solvent-driven structural changes in anion-π complexes. J Chem Theory Comput 4:1162–1169
Dawson RE, Hennig A, Weimann DP, Emery D, Ravikumar V, Montenegro J, Takeuchi T, Gabutti S, Mayor M, Mareda J, Schalley CA, Matile S (2010) Experimental evidence for the functional relevance of anion-π interactions. Nat Chem 2:533–538
Ballester P (2013) Experimental quantification of anion-π interactions in solution using neutral host-guest model systems. Acc Chem Res 46:874–884
Hay BP, Custelcean R (2009) Anion-π interactions in crystal structures: commonplace or extraordinary? Cryst Growth Des 9:2539–2545
Demeshko S, Dechert S, Meyer F (2004) Anion−π interactions in a carousel copper(II)−triazine complex. J Am Chem Soc 126:4508–4509
Rosokha YS, Lindeman SV, Rosokha SV, Kochi JK (2004) Halide recognition through diagnostic“anion-π” interactions: molecular complexes of Cl-, Br-, and I- with olefinic and aromatic π receptors. Angew Chem Int Ed 43:4650–4652
de Hoog P, Gamez P, Mutikainen I, Turpeinen U, Reedijk J (2004) An aromatic anion receptor: anion-π interactions Do exist. Angew Chem Int Ed 43:5815–5817
Schottel BL, Bacsa J, Dunbar KR (2005) Anion dependence of Ag(I) reactions with 3,6-Bis(2-pyridyl)-1,2,4,5-tetrazine (bptz): isolation of the molecular propeller compound [Ag2(bptz)3][AsF6]2. Chem Commun 46–47
Wang D-X, Zheng Q-Y, Wang Q-Q, Wang M-X (2008) Halide recognition by tetraoxacalix[2]arene[2]triazine receptors: concurrent noncovalent halide-π and lone-pair-π interactions in host−halide−water ternary complexes. Angew Chem Int Ed 47:7485–7488
Wang D-X, Wang Q-Q, Han Y, Wang Y, Huang Z-T, Wang M-X (2010) Versatile anion-π interactions between halides and a conformationally rigid Bis(tetraoxacalix[2]arene[2]triazine) cage and their directing effect on molecular assembly. Chem Eur J 16:13053–13057
Das A, Choudhury SR, Estarellas C, Dey B, Frontera A, Hemming J, Helliwell M, Gamez P, Mukhopadhyay S (2011) Supramolecular assemblies involving anion–π and lone pair–π interactions: experimental observation and theoretical analysis. CrystEngComm 13:4519–4527
Lucas X, Estarellas C, Escudero D, Frontera A, Quinonero D, Deya PM (2009) Very long-range effects: cooperativity between anion-π and hydrogen-bonding interactions. ChemPhysChem 10:2256–2264
Escudero D, Frontera A, Quinonero D, Deya PM (2009) Interplay between anion-π and hydrogen bonding interactions. J Comput Chem 30:75–82
Quinonero D, Frontera A, Garau C, Ballester P, Costa A, Deya PM (2006) Interplay between cation–π, anion–π and π–π interactions. ChemPhysChem 7:2487–2491
Frontera A, Quinonero D, Costa A, Ballester P, Deya PM (2007) MP2 study of cooperative effects between cation–π, anion–π and π–π interactions. New J Chem 31:556–560
Estarellas C, Frontera A, Quinonero D, Deya PM (2011) Theoretical study on cooperativity effects between anion–π and halogen-bonding interactions. ChemPhysChem 12:2742–2750
Berryman OB, Bryantsev VS, Stay DP, Johnson DW, Hay BP (2007) Structural criteria for the design of anion receptors: the interaction of halides with electron-deficient arenes. J Am Chem Soc 129:48–58
Hay BP, Bryantsev VS (2008) Anion-arene adducts: C-H hydrogen bonding, anion-π interaction, and carbon bonding motifs. Chem Commun 21:2417–2428
Mascal M, Yakovlev I, Nikitin EB, Fettinger JC (2007) Fluoride-selective host based on anion-π interactions, ion pairing, and hydrogen bonding: synthesis and fluoride-ion sandwich complex. Angew Chem Int Ed 46:8782–8784
Alberto ME, Mazzone G, Russo N, Sicilia E (2010) The mutual influence of Non-covalent interactions in π-electron deficient cavities: the case of anion recognition by tetraoxacalix[2]-arene[2]triazine. Chem Commun 46:5894–5896
Chen Y-S (2013) Theoretical study of interactions between halogen-substituted s-triazine and halide anions. J Phys Chem A 117:8081–8090
Chen Y-S, Yao L-F (2014) Theoretical study of X- · 1 · YF (1 = triazine, X = Cl, Br and I, Y = H, Cl, Br, I, PH2 and AsH2): noncovalently electron-withdrawing effects on anion-arene interactions. J Mol Model 2076, 1−11
Schneider H, Boese AD, Weber J (2005) Unusual hydrogen bonding behavior in binary complexes of coinage metal anions with water. J Chem Phys 123:084307, 1−6
Kryachko ES, Remacle F (2007) The gold-ammonia bonding patterns of neutral and charged complexes Aum 0±1–(NH3)n. I. Bonding and charge alternation. J Chem Phys 127:194305, 1−11
Cao G-J, Xu H-G, Li R-Z, Zheng W-J (2012) Hydrogen bonds in the nucleobase-gold complexes: photoelectron spectroscopy and density functional calculations. J Chem Phys 136:014305, 1−8
Vargas R, Martínez A (2011) Non-conventional hydrogen bonds: pterins-metal anions. Phys Chem Chem Phys 13:12775–12784
Frisch MJ et al (2010) Gaussian 09. Gaussian Inc, Wallingford, CT
Peterson KA, Puzzarini C (2005) Systematically convergent basis sets for transition metals. II. Pseudopotential-based correlation consistent basis sets for the group 11 (Cu, Ag, Au) and 12 (Zn, Cd, Hg) elements. Theor Chem Acc 114:283–296
Boys SF, Bernardi F (1970) Calculation of small molecular interactions by differences of separate total energies – some procedures with reduced errors. Mol Phys 19:553–566
Alvarez-Idaboy JR, Galano A (2010) Counterpoise corrected interaction energies are not systematically better than uncorrected ones: comparison with CCSD(T) CBS extrapolated values. Theor Chem Acc 126:75–85
Mentel LM, Baerend EJ (2014) Can the counterpoise correction for basis set superposition effect be justified? J Chem Theory Comput 10:252–267
Weinhold F, Landis C (2012) Discovering chemistry with natural bond orbitals. Wiley, Hoboken
Dennington R, Keith T, Millam JS (2009) GaussView, version 5. Semichem Inc, Shawnee Mission, KS
Wiberg KB (1968) Application of the pople-santry-Segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24:1083–1096
Dick B, Freund HJ (1983) Analysis of bonding properties in molecular ground and excited states by a Cohen-type bond order. Int J Quantum Chem 24:747–765
Acknowledgments
Supported by the HPC Center, Kunming Institute of Botany, CAS, China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 132 kb)
Rights and permissions
About this article
Cite this article
Chen, Y., Wang, F. Theoretical study of interactions between electron-deficient arenes and coinage metal anions. J Mol Model 21, 38 (2015). https://doi.org/10.1007/s00894-015-2584-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2584-6