Abstract
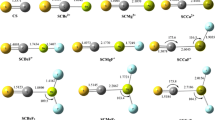
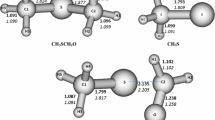
In this work ab initio calculations at MP2 level in conjugation with aug-cc-pVXZ (X=D and T) basis set were performed in order to examine complexes formed between SO4 and each of linear molecules of CO2, CS2, and SCO. The results have been discussed on real minima located on singlet potential energy surface (PES). Single-point energy calculations at the MP2/aug–cc–pVTZ level uphold results obtained at the MP2/aug–cc–pVDZ level. The atom in molecules theory (AIM) was utilized to analyze the nature of intermolecular interactions. Also, natural bond orbital (NBO) analysis has been used in order to get charge transfer quota in complexes. The results show that the atmospheric role of SO4–CS2 system is more important than those followed by SO4–SCO and SO4-CO2, respectively.



Similar content being viewed by others
References
Scheiner S (2009) J Phys Chem B 113:10421
Muller-Dethlefs K, Hobza P (2000) Chem Rev 100:143
Farquhar J, Bao H, Thiemens M (2000) Science 289:756
Habicht KS, Gade M, Thamdrup B, Berg P, Canfield DE (2002) Science 298:2372
Kugel R, Taube H (1975) J Phys Chem 79:2130
McKee ML (1993) J Am Chem Soc 115:9136
McKee ML (1996) J Phys Chem 100:3473
Goodarzi M, Vahedpour M, Solimannejad M (2012) Chem Phys Lett 538:10
Wannagat V, Schwarz R (1956) Z Anorg Allg Chem 286:180
Goodarzi M, Vahedpour M, Solimannejad M (2012) Struct Chem 23:1609
Sidebottom HW, Badcock CC, Jackson GE, Calvert JG, Teinhardt GW, Damon EK (1972) Environ Sci Technol 6:72
Allen ER, McQuigg RD, Cadle RD (1972) Chemosphere 1:25
Cox RA (1972) J Phys Chem 76:814
Vohra KG, Nair PVN, Muraieedharan TS (1972) J Aerosol Sci 3:225
Mark C, Davis DD (1993) Glob Biogeochem Cycles 7:321
Thornton DC, Bandy AR, Blomquist BW (1996) J Geophys Res 101:1873
Seinfeld J (2006) Atmospheric chemistry and physics. Wiley, London
Møller C, Plesset MS (1934) Phys Rev 46:618
Dunning TH (1989) J Chem Phys 90:1007
Dunning TH, Peterson KA, Wilson AK (2001) J Chem Phys 114:9244
Boys SF, Bernardi F (1970) Mol Phys 19:553
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) Gamess Version 11. J Comput Chem 14:1347
Bulat F, Toro-Labbé A, Brinck T, Murray J, Politzer P (2010) J Mol Model 16:1679
Bader RFW, Carroll MT, Cheeseman JR, Chang C (1987) J Am Chem Soc 109:7968
Bader RFW (1990) In: Halpen J, Green MLH (eds) The international series of monographs of chemistry. Clarendon Press, Oxford
Biegler-Konig F, Schonbohm J (2002) AIM2000 Program Package, Ver. 2.0. University of Applied Sciences, Bielefeld
Sloss LL (1992) Nitrogen oxides control technology fact book. William Andrew, Norwich, p 6
Curtiss L, Pochatko DG, Reed AE, Weinhold F (1985) J Chem Phys 82:2679
Reed AE, Weinhold F (1985) J Chem Phys 83:1736
Foster JP, Weinhold F (1980) J Am Chem Soc 102:7211
Seif A, Goodarzi M (2014) Struct Chem 25:941
Ziołkowski M, Grabowski SJ, Leszczynski J (2006) J Phys Chem A 110:6514
Seif A, Bagherzadeh R, Goodarzi M, Azizi K (2013) J Chem Sci 125:1277
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Seif, A., Massahi, S. Theoretical study on the properties and stabilities of complexes formed between SO4 (C2v) and isostructure species of CO2, CS2, and SCO. J Mol Model 20, 2488 (2014). https://doi.org/10.1007/s00894-014-2488-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2488-x