Abstract
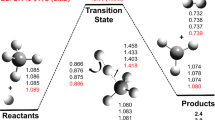
The mechanism of Menshutkin reaction, NH3 + CH3Cl = [CH3–NH3]+ + Cl-, has been thoroughly studied in both gas and solvent (H2O and cyclohexane) phase. It has been found that solvents favor the reaction, both thermodynamically and kinetically. The electronic activity that drives the mechanism of the reaction was identified, fully characterized, and associated to specific chemical events, bond forming/breaking processes, by means of the reaction electronic flux. This led to a complete picture of the reaction mechanism that was independently confirmed by natural bond-order analysis and the dual descriptor for chemical reactivity and selectivity along the reaction path.






Similar content being viewed by others
References
Menshutkin NA (1890) Z Phys Chem 6:41
Menshutkin N (1890) Z Phys Chem 5:589
Abboud JLM, Notario R, Bertran J, Solá M (1993) In onlinelibrary.wiley.com; Progress in Physical Organic Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA; Vol. 19, pp. 1–182
Pedley JB. Thermochemical data and structures of organic compounds, 18 ed. worldcat.org: College Station, TX, USA
Viers JW, Schug JC, Stovall MD, Seeman JIJ (1984) Comput Chem 5:598–605
Chandrasekhar J, Jorgensen WL (1985) J Am Chem Soc 107:2974
Solà M, Lledós A, Duran M, Bertran J, Abboud JLMJ (1991) J Am Chem Soc 113:2873–2879
Gao J (1991) J Am Chem Soc
Maran U, Pakkanen TA, Karelson MJ (1994) Chem Soc Perkin Trans 2:2445–2452
Shaik S, Ioffe A, Reddy AC, Pross AJ (1994) J Am Chem Soc 116:262–273
Fradera X, Amat L, Torrent M, Mestres J, Constans P, Besalú E, Martí J, Simon S, Lobato M, Oliva JM, Luis JM, Andrés JL, Solá M, Carbó R, Duran M (1996) J Mol Struct (THEOCHEM) 371:171–183
Truong TN, Truong T-TT, Stefanovich EV (1997) J Chem Phys 107:1881–1889
Amovilli C, Mennucci B, Floris FMJ (1998) Phys Chem B 102:3023–3028
Melo A, Alfaia AJ, Reis JCR, Calado ARJ (2006) Phys Chem B 110:1877–1888
Cox HE (1921) J Chem Soc Trans 119, 142
Grimm HG, Ruford H, Wolff H (1931) Z Physik Chem 1353
Pickles NJT, Hinshelwood CN (1936) J Chem Soc B 1353
Raine HC, Hinshelwood CN (1939) J Chem Soc 1378
Tommila E, Murto ML (1963) Acta Chem Scand
Arnett EM, Reich RJ (1980) Am Chem Soc 102:5892–5902
Jay AN, Daniel KA, Patterson EVJ (2007) Chem Theory Comput 3:336–343
Bulat FA, Toro-Labbé A (2003) J Phys Chem A 107:3987–3994
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999–3094
Toro-Labbé A (1999) J Phys Chem A 103:4398–4403
Gutiérrez-Oliva S, Herrera B, Toro-Labbé A, Chermette H (2005) J Phys Chem A 109:1748–1751
Herrera B, Toro-Labbé A (2007) J Phys Chem A 111:5921–5926
Herrera B, Toro-Labbé A (2004) J Chem Phys 121:7096–7102
Marcus RA (1964) Annu Rev Phys Chem 15:155–196
Leffler JE (1953) Science 117:340–341
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Geerlings P, De Proft F, Langenaeker W (2003) Chem Rev 103:1793
Anderson JSM, Melin J, Ayers PWJ (2007) Chem Theory Comput 3:358–374
Wu Q, Ayers PW, Yang W (2003) J Chem Phys 119:2978
Ayers PW, Parr RG, Pearson RG (2006) J Chem Phys 124:194107
Johnson PA, Solà M, Bartolotti LJ, Solà M, Ayers PW, Lledós A, Fievez T, Lledós A, Duran M, Bertran J, Abboud JLM (2012) Vol. 1, pp. 715–764
Bartolotti LJ, Ayers PW (2005) J Phys Chem A 109:1146–1151
Morell C, Grand A, e AT-L (2005) J Phys Chem A 109, 205
Morell C, Grand A, Toro-Labbé A (2006) Chem Phys Lett 425:342–346
Ayers PW, Morell C, De Proft F, Geerlings P (2007) Chem Eur J 13:8240–8247
Morel C, Grand A, Gutierrez-Oliva S, Toro-Labbé A. In Theoretical Aspects of Chemical Reactivity; Toro-Labbé A (ed) Elsevier, Oxford, pp 101–117
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Echegaray E, Gutierrez-Oliva S, Herrera B (2011) Science China
Duarte F, Toro-Labbé A (2010) Mol Phys 108:1375–1384
Fukui K (1970) J Phys Chem 74:4161–4163
Fukui K (1981) Acc Chem Res 14:363–368
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154–2161
Hratchian HP, Nelson KV, Viers JW, Schlegel HB, Benjamin I, Viers JW, Schug JC, Schug JC, Stovall MD, Stovall MD, Seeman JI, Seeman JI (2004) J Chem Phys 120:9918
Hratchian HP, Schlegel HBJ (2005) Chem Theory Comput 1:61–69
Toro-Labbé A, Gutiérrez-Oliva S, Murray JS, Politzer P (2008) J Mol Model 15:707–710
Politzer P, Toro-Labbé A, Gutiérrez-Oliva S, Herrera B, Jaque P, Concha MC, Murray JS (2005) J Chem Sci 117:467–472
Echegaray E, Toro-Labbé A (2008) J Phys Chem A 112:11801–11807
Vogt-Geisse S, Toro-Labbé A (2009) J Chem Phys 130:244308
Rincón E, Jaque P, Toro-Labbé A (2006) J Phys Chem A 110:9478–9485
Politzer P, Burda JV, Concha MC, Lane P, Murray JS (2006) J Phys Chem A 110:756–761
Sen KD, Jorgensen CK (1987) Electronegativity: structure and bonding, vol 66. Springer Verlag, Berlin
Parr RG, Donnelly RA, Levy M, Palke WE (1978) J Chem Phys 68:3801
Koopmans T (1934) Physica 1:104–113
Parr RG, Yang WJ (1984) Am Chem Soc 106:4049–4050
Morell C, Grand A, Toro-Labbé A (2005) J Phys Chem A 109:205–212
Zhao Y, Truhlar DGJ (2006) Chem Theory Comput 2:1009–1018
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Frisch H, Trucks MJ, Schlegel GW, Scuseria HB, Robb GE, Cheeseman MA, Scalmani JR, Barone G, Mennucci V, Petersson B, Nakatsuji GA, Caricato H, Li M, Hratchian X, Izmaylov HP, Bloino AF, Zheng J, Sonnenberg G, Hada JL, Ehara M, Toyota M, Fukuda K, Hasegawa R, Ishida J, Nakajima M, Honda T, Kitao Y, Nakai O, Vreven H, Montgomery TJ, Peralta JA, Ogliaro JE, Bearpark F, Heyd M, Brothers JJ, Kudin E, Staroverov KN, Kobayashi VN, Normand R, Raghavachari J, Rendell K, Burant A, Iyengar JC, Tomasi SS, Cossi J, Rega M, Millam N, Klene JM, Knox M, Cross JE, Bakken JB, Adamo V, Jaramillo C, Gomperts J, Stratmann R, Yazyev RE, Austin O, Cammi AJ, Pomelli R, Ochterski C, Martin JW, Morokuma RL, Zakrzewski K, Voth VG, Salvador GA, Dannenberg P, Dapprich JJ, Daniels S, Farkas AD, Foresman Ö, Ortiz JB, Cioslowski JV, Fox J (2009) Gaussian 09, 1st edn. Gaussian Inc, Wallingford
Acknowledgments
This work was supported by FONDECYT through projects N°1120093 and N°1130072. The authors acknowledge financial support from ICM through project N° 120082 S. RIR wishes to thank CONICYT for Doctoral and Apoyo de Tesis Fellowships.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection QUITEL 2013
Rights and permissions
About this article
Cite this article
Giri, S., Inostroza-Rivera, R., Herrera, B. et al. The mechanism of Menshutkin reaction in gas and solvent phases from the perspective of reaction electronic flux. J Mol Model 20, 2353 (2014). https://doi.org/10.1007/s00894-014-2353-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2353-y