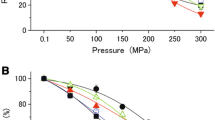
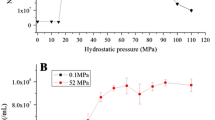
Abstract
In piezophilic microorganisms, enzymes are optimized to perform under high hydrostatic pressure. The two major reported mechanisms responsible for such adaptation in bacterial species are changes in amino acids in the protein structure, favoring their activity and stability under high-pressure conditions, and the possible accumulation of micromolecular co-solutes in the cytoplasm. Recently, the accumulation of glutamate in the cytoplasm of piezophilic Desulfovibrio species has been reported under high-pressure growth conditions. In this study, analysis of the effect of glutamate on the enzymatic activity of the thioredoxin reductase/thioredoxin enzymatic complex of either a piezosensitive or a piezophilic microorganism confirms its role as a protective co-solute. Analysis of the thioredoxin structures suggests an adaptation both to the presence of glutamate and to high hydrostatic pressure in the enzyme from the piezophilic strain. Indeed, the presence of large surface pockets could counterbalance the overall compression that occurs at high hydrostatic pressure to maintain enzymatic activity. A lower isoelectric point and a greater dipolar moment than that of thioredoxin from the piezosensitive strain would allow the protein from the piezophilic strain to compensate for the presence of the charged amino acid glutamate to interact with its partner.




Similar content being viewed by others
References
Abe F (2007) Exploration of the effects of high hydrostatic pressure on microbial growth, physiology and survival: perspectives from piezophysiology. Biosci Biotechnol Biochem 71:2347–2357. https://doi.org/10.1271/bbb.70015
Amrani A, Bergon A, Holota H, Tamburini C, Garel M, Ollivier B, Imbert J, Dolla A, Pradel N (2014) Transcriptomics reveal several gene expression patterns in the piezophile Desulfovibrio hydrothermalis in response to hydrostatic pressure. PLoS ONE 9:e106831. https://doi.org/10.1371/journal.pone.0106831
Amrani A, van Helden J, Bergon A, Aouane A, Ben Hania W, Tamburini C, Loriod B, Imbert J, Ollivier B, Pradel N, Dolla A (2016) Deciphering the adaptation strategies of Desulfovibrio piezophilus to hydrostatic pressure through metabolic and transcriptional analyses. Environ Microbiol Rep 8:520–526. https://doi.org/10.1111/1758-2229.12427
Arakawa T, Timasheff SN (1985) The stabilization of proteins by osmolytes. Biophys J 47:411–414. https://doi.org/10.1016/S0006-3495(85)83932-1
Cornilescu G, Delaglio F, Bax A (1999) Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J Biomol NMR 13:289–302. https://doi.org/10.1023/a:1008392405740
Crowe JH, Carpenter JF, Crowe LM, Anchordoguy TJ (1990) Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology 27:219–231. https://doi.org/10.1016/0011-2240(90)90023-w
Cuff AL, Martin AC (2004) Analysis of void volumes in proteins and application to stability of the p53 tumour suppressor protein. J Mol Biol 344:1199–1209. https://doi.org/10.1016/j.jmb.2004.10.015
Eiberweiser A, Nazet A, Kruchinin SE, Fedotova MV, Buchner R (2015) Hydration and ion binding of the osmolyte ectoine. J Phys Chem B 119:15203–15211. https://doi.org/10.1021/acs.jpcb.5b09276
Fedotova M, Kruchinin S, Chuev G (2017) Hydration structure of osmolyte TMAO: concentration/pressure-induced response. New J Chem 41:1219–1228. https://doi.org/10.1039/C6NJ03296F
Gross M, Jaenicke R (1994) Proteins under pressure. The influence of high hydrostatic pressure on structure, function and assembly of proteins and protein complexes. Eur J Biochem 221:617–630. https://doi.org/10.1111/j.1432-1033.1994.tb18774.x
Hamajima Y, Nagae T, Watanabe N, Ohmae E, Kato-Yamada Y, Kato C (2016) Pressure adaptation of 3-isopropylmalate dehydrogenase from an extremely piezophilic bacterium is attributed to a single amino acid substitution. Extremophiles 20:177–186. https://doi.org/10.1007/s00792-016-0811-4
Huang Q, Rodgers J, Hemley R, Ichiye T (2019) Effects of pressure and temperature on the atomic fluctuations of dihydrofolate reductase from a psychropiezophile and a mesophile. IJMS 20:1452–1465. https://doi.org/10.3390/ijms20061452
Kang Y, Hwang I (2018) Glutamate uptake is important for osmoregulation and survival in the rice pathogen Burkholderia glumae. PLoS ONE 13:e0190431. https://doi.org/10.1371/journal.pone.0190431
Katti SK, LeMaster DM, Eklund H (1990) Crystal structure of thioredoxin from Escherichia coli at 1.68 Å resolution. J Mol Biol 212:167–184. https://doi.org/10.1016/0022-2836(90)90313-B
Keller RLJ (2004) The computer aided resonance assignment tutorial. Cantina Verlag, Goldau
Le Bihan T, Rayner J, Roy MM, Spagnolo L (2013) Photobacterium profundum under pressure: a MS-based label-free quantitative proteomics study. PLoS ONE 8:e60897. https://doi.org/10.1371/journal.pone.0060897
Martin J (1995) Thioredoxin–a fold for all reasons. Structure 3:245–250. https://doi.org/10.1016/s0969-2126(01)00154-x
Martin D, Bartlett D, Roberts M (2002) Solute accumulation in the deep-sea bacterium Photobacterium profundum. Extremophiles 6:507–514. https://doi.org/10.1007/s00792-002-0288-1
Pieulle L, Stocker P, Vinay M, Nouailler M, Vita N, Brasseur G, Garcin E, Sebban-Kreuzer C, Dolla A (2011) Study of the thiol/disulfide redox systems of the anaerobe Desulfovibrio vulgaris points out pyruvate:ferredoxin oxidoreductase as a new target for thioredoxin 1. J Biol Chem 286:7812–7821. https://doi.org/10.1074/jbc.M110.197988
Robinson CR, Sligar SG (1994) Hydrostatic pressure reverses osmotic pressure effects on the specificity of EcoRI-DNA interactions. Biochemistry 33:3787–3793. https://doi.org/10.1021/bi00179a001
Robinson CR, Sligar SG (1995) Hydrostatic and osmotic pressure as tools to study macromolecular recognition. Methods Enzymol 259:395–427. https://doi.org/10.1016/0076-6879(95)59054-4
Sambrook J, Fritsch EF, Maniatis T (1989) Bacterial media, antibiotics and bacterial strains. In: C N (ed) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York, pp A1–A13
Sarma R, Paul S (2013) Crucial importance of water structure modification on trimethylamine N-oxide counteracting effect at high pressure. J Phys Chem B 117:677–689. https://doi.org/10.1021/jp311102v
Saum SH, Müller V (2007) Salinity-dependent switching of osmolyte strategies in a moderately halophilic bacterium: glutamate induces proline biosynthesis in Halobacillus halophilus. J Bacteriol 189:6968–6975. https://doi.org/10.1128/JB.00775-07
Scoma A, Barbato M, Borin S, Daffonchio D, Boon N (2016) An impaired metabolic response to hydrostatic pressure explains Alcanivorax borkumensis recorded distribution in the deep marine water column. Sci Rep 6:31316. https://doi.org/10.1038/srep31316
Tian W, Chen C, Lei X, Zhao J, Liang J (2018) CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res 46:W363–W367. https://doi.org/10.1093/nar/gky473
Valette O, Tran TTT, Cavazza C, Caudeville E, Brasseur G, Dolla A, Talla E, Pieulle L (2017) Biochemical function, molecular structure and evolution of an atypical thioredoxin reductase from Desulfovibrio vulgaris. Front Microbiol 8:1855. https://doi.org/10.3389/fmicb.2017.01855
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46:W296–W303. https://doi.org/10.1093/nar/gky427
Xie Z, Jian H, Jin Z, Xiao X (2018) Enhancing the adaptability of the deep-sea bacterium Shewanella piezotolerans WP3 to high pressure and low temperature by experimental evolution under H2O2 stress. Appl Environl Microbiol 84:e02342-e2417. https://doi.org/10.1128/AEM.02342-17
Yancey PH, Siebenaller JF (2015) Co-evolution of proteins and solutions: protein adaptation versus cytoprotective micromolecules and their roles in marine organisms. J Exp Biol 218:1880–1896. https://doi.org/10.1242/jeb.114355
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by A. Driessen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gaussier, H., Nouailler, M., Champaud, E. et al. Glutamate optimizes enzymatic activity under high hydrostatic pressure in Desulfovibrio species: effects on the ubiquitous thioredoxin system. Extremophiles 25, 385–392 (2021). https://doi.org/10.1007/s00792-021-01236-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-021-01236-x