Abstract
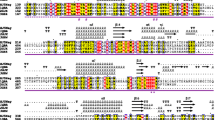
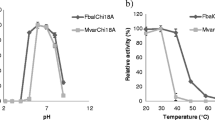
β-N-Acetylglucosaminidases (GlcNAcases) are important for many biological functions and industrial applications. In this study, a glycoside hydrolase family 20 GlcNAcase from Shinella sp. JB10 was expressed in Escherichia coli BL21 (DE3). Compared to many GlcNAcases, the purified recombinant enzyme (rJB10Nag) exhibited a higher specificity activity (538.8 µmol min−1 mg−1) or V max (1030.0 ± 82.1 µmol min−1 mg−1) toward p-nitrophenyl β-N-acetylglucosaminide and N,N′-diacetylchitobiose (specificity activity of 35.4 µmol min−1 mg−1) and a higher N-acetylglucosaminide tolerance (approximately 50% activity in 70.0 mM N-acetylglucosaminide). The degree of synergy on enzymatic degradation of chitin by a commercial chitinase and rJB10Nag was as high as 2.35. The enzyme was tolerant to most salts, especially 3.0–15.0% (w/v) NaCl and KCl. These biochemical characteristics make the JB10 GlcNAcase a candidate for use in many potential applications, including processing marine materials and the bioconversion of chitin waste. Furthermore, the enzyme has the highest proportions of alanine (16.5%), glycine (10.5%), and random coils (48.8%) with the lowest proportion of α-helices (24.9%) among experimentally characterized GH 20 GlcNAcases from other organisms.





Similar content being viewed by others
References
Bhabha G, Lee J, Ekiert DC, Gam J, Wilson IA, Dyson HJ, Benkovic SJ, Wright PE (2011) A dynamic knockout reveals that conformational fluctuations influence the chemical step of enzyme catalysis. Science 332:234–238
Bruce AF, Gounaris K (2006) Characterisation of a secreted N-acetyl-β-hexosaminidase from Trichinella spiralis. Mol Biochem Parasit 145:84–93
Chen F, Chen XZ, Qin LN, Tao Y, Dong ZY (2015) Characterization and homologous overexpression of an N-acetylglucosaminidase Nag1 from Trichoderma reesei. Biochem Bioph Res Co 459:184–188
Chitlaru E, Roseman S (1996) Molecular cloning and characterization of a novel β-N-acetyl-d-glucosaminidase from Vibrio furnissii. J Biol Chem 271:33433–33439
Choi KH, Seo JY, Park KM, Park CS, Cha J (2009) Characterization of glycosyl hydrolase family 3 β-N-acetylglucosaminidases from Thermotoga maritima and Thermotoga neapolitana. J Biosci Bioeng 108:455–459
da Silva Junior Sobrinho I, Bataus LAM, Maitan VR, Ulhoa CJ (2005) Purification and properties of an N-acetylglucosaminidase from Streptomyces cerradoensis. Biotechnol Lett 27:1273–1276
Hong SY, Yoo YJ (2013) Activity enhancement of Candida antarctica lipase B by flexibility modulation in helix region surrounding the active site. Appl Biochem Biotech 170:925–933
Hong SY, Park HJ, Yoo YJ (2014) Flexibility analysis of activity-enhanced mutants of bacteriophage T4 lysozyme. J Mol Catal B-Enzym 106:95–99
Inokuma K, Hasunuma T, Kondo A (2016) Ethanol production from N-acetyl-d-glucosamine by Scheffersomyces stipitis strains. AMB Express 6:83
Keyhani NO, Roseman S (1996) The chitin catabolic cascade in the marine bacterium Vibrio furnissii—molecular cloning, isolation, and characterization of a periplasmic β-N-acetylglucosaminidase. J Biol Chem 271:33425–33432
Kubota T, Miyamoto K, Yasuda M, Inamori Y, Tsujibo H (2004) Molecular characterization of an intracellular β-N-acetylglucosaminidase involved in the chitin degradation system of Streptomyces thermoviolaceus OPC-520. Biosci Biotech Bioch 68:1306–1314
Lan XQ, Ozawa N, Nishiwaki N, Kodaira R, Okazaki M, Shimosaka M (2004) Purification, cloning, and sequence analysis of β-N-acetylglucosaminidase from the chitinolytic bacterium Aeromonas hydrophila strain SUWA-9. Biosci Biotech Bioch 68:1082–1090
Lemieux MJ, Mark BL, Cherney MM, Withers SG, Mahuran DJ, James MNG (2006) Crystallographic structure of human β-hexosaminidase A: interpretation of Tay-Sachs mutations and loss of G(M2) ganglioside hydrolysis. J Mol Biol 359:913–929
Lin H, Xiao X, Zeng X, Wang FP (2006) Expression, characterization and mutagenesis of the gene encoding β-N-acetylglucosaminidase from Aeromonas caviae CB101. Enzyme Microb Tech 38:765–771
Liu TA, Zhang HT, Liu FY, Wu QY, Shen X, Yang Q (2011) Structural determinants of an insect β-N-acetyl-d-hexosaminidase specialized as a chitinolytic enzyme. J Biol Chem 286:4049–4058
Madern D, Ebel C, Zaccai G (2000) Halophilic adaptation of enzymes. Extremophiles 4:91–98
Margesin R, Schinner F (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83
Mark BL, Wasney GA, Salo TJS, Khan AR, Cao ZM, Robbins PW, James MNG, Triggs-Raine BL (1998) Structural and functional characterization of Streptomyces plicatus β-N-acetylhexosaminidase by comparative molecular modeling and site-directed mutagenesis. J Biol Chem 273:19618–19624
Matsui T, Shinzato N, Tamaki H, Muramatsu M, Hanada S (2009) Shinella yambaruensis sp. nov., a 3-methyl-sulfolane-assimilating bacterium isolated from soil. Int J Syst Evol Micr 59:536–539
Meekrathok P, Suginta W (2016) Probing the catalytic mechanism of Vibrio harveyi GH20 β-N-acetylglucosaminidase by chemical rescue. PLoS One 11:e0149228
Molina-Guijarro JM, Perez J, Munoz-Dorado J, Guillen F, Moya R, Hernandez M, Arias ME (2009) Detoxification of azo dyes by a novel pH-versatile, salt-resistant laccase from Streptomyces ipomoea. Int Microbiol 12:13–21
Nieder V, Kutzer M, Kren V, Gallego RG, Kamerling JP, Elling L (2004) Screening and characterization of β-N-acetylhexosaminidases for the synthesis of nucleotide-activated disaccharides. Enzyme Microb Tech 34:407–414
O’Connell E, Murray P, Piggott C, Hennequart F, Tuohy M (2008) Purification and characterization of a N-acetylglucosaminidase produced by Talaromyces emersonii during growth on algal fucoidan. J Appl Phycol 20:557–565
Ogawa M, Kitagawa M, Tanaka H, Ueda K, Watsuji T, Beppu T, Kondo A, Kawachi R, Oku T, Nishio T (2006) A β-N-acetylhexosaminidase from Symbiobacterium thermophilum; gene cloning, overexpression, purification and characterization. Enzyme Microb Tech 38:457–464
Ohishi K, Murase K, Etoh H (1999) Purification and properties of β-N-acetylglucosaminidase from Vibrio alginolyticus H-8. J Biosci Bioeng 88:98–99
Park JK, Kim WJ, Park YI (2011) Purification and characterization of an exo-type β-N-acetylglucosaminidase from Pseudomonas fluorescens JK-0412. J Appl Microbiol 110:277–286
Patil RS, Ghormade V, Deshpande MV (2000) Chitinolytic enzymes: an exploration. Enzyme Microb Tech 26:473–483
Paul S, Bag SK, Das S, Harvill ET, Dutta C (2008) Molecular signature of hypersaline adaptation: insights from genome and proteome composition of halophilic prokaryotes. Genome Biol 9:R70
Premkumar L, Greenblatt HM, Bageshwar UK, Savchenko T, Gokhman I, Sussman JL, Zamir A (2005) Three-dimensional structure of a halotolerant algal carbonic anhydrase predicts halotolerance of a mammalian homolog. P Natl Acad Sci USA 102:7493–7498
Qin YJ, Huang ZQ, Liu ZD (2014) A novel cold-active and salt-tolerant α-amylase from marine bacterium Zunongwangia profunda: molecular cloning, heterologous expression and biochemical characterization. Extremophiles 18:271–281
Qiu JG, Wei Y, Ma Y, Wen RT, Wen YZ, Liu WP (2014) A novel (S)-6-hydroxynicotine oxidase gene from Shinella sp. strain HZN7. Appl Environ Microb 80:5552–5560
Riekenberg S, Flockenhaus B, Vahrmann A, Muller MCM, Leippe M, Kiess M, Scholze H (2004) The β-N-acetylhexosaminidase of Entamoeba histolytica is composed of two homologous chains and has been localized to cytoplasmic granules. Mol Biochem Parasit 138:217–225
Senba M, Kashige N, Nakashima K, Miake F, Watanabe K (2000) Cloning of the gene of β-N-acetylglucosaminidase from Lactobacillus casei ATCC 27092 and characterization of the enzyme expressed in Escherichia coli. Biol Pharm Bull 23:527–531
Shen JD, Zhang R, Li JJ, Tang XH, Li RX, Wang M, Huang ZX, Zhou JP (2015) Characterization of an exo-inulinase from Arthrobacter: a novel NaCl-tolerant exo-inulinase with high molecular mass. Bioengineered 6:99–105
Slamova K, Kulik N, Fiala M, Krejzova-Hofmeisterova J, Ettrich R, Kren V (2014) Expression, characterization and homology modeling of a novel eukaryotic GH84 β-N-acetylglucosaminidase from Penicillium chrysogenum. Protein Expres Purif 95:204–210
Suginta W, Chuenark D, Mizuhara M, Fukamizo T (2010) Novel β-N-acetylglucosaminidases from Vibrio harveyi 650: cloning, expression, enzymatic properties, and subsite identification. BMC Biochem 11:40
Sumida T, Ishii R, Yanagisawa T, Yokoyama S, Ito M (2009) Molecular cloning and crystal structural analysis of a novel β-N-acetylhexosaminidase from Paenibacillus sp. TS12 capable of degrading glycosphingolipids. J Mol Biol 392:87–99
Tews I, Perrakis A, Oppenheim A, Dauter Z, Wilson KS, Vorgias CE (1996) Bacterial chitobiase structure provides insight into catalytic mechanism and the basis of Tay-Sachs disease. Nat Struct Mol Biol 3:638–648
Tomiya N, Narang S, Park J, Abdul-Rahman B, Choi O, Singh S, Hiratake J, Sakata K, Betenbaugh MJ, Palter KB, Lee YC (2006) Purification, characterization, and cloning of a Spodoptera frugiperda Sf9 β-N-acetylhexosaminidase that hydrolyzes terminal N-acetylglucosamine on the N-glycan core. J Biol Chem 281:19545–19560
Tsujibo H, Hatano N, Mikami T, Hirasawa A, Miyamoto K, Inamori Y (1998) A novel β-N-acetylglucosaminidase from Streptomyces thermoviolaceus OPC-520: gene cloning, expression, and assignment to family 3 of the glycosyl hydrolases. Appl Environ Microb 64:2920–2924
Wang GZ, Wang QH, Lin XJ, Ng TB, Yan RX, Lin J, Ye XY (2016) A novel cold-adapted and highly salt-tolerant esterase from Alkalibacterium sp. SL3 from the sediment of a soda lake. Sci Rep-UK 6:19494
Warden AC, Williams M, Peat TS, Seabrook SA, Newman J, Dojchinov G, Haritos VS (2015) Rational engineering of a mesohalophilic carbonic anhydrase to an extreme halotolerant biocatalyst. Nat Commun 6:10278
Yang SQ, Song S, Yan QJ, Fu X, Jiang ZQ, Yang XB (2014) Biochemical characterization of the first fungal glycoside hydrolyase family 3 β-N-acetylglucosaminidase from Rhizomucor miehei. J Agr Food Chem 62:5181–5190
Zhou JP, Zhang R, Gao YJ, Li JJ, Tang XH, Mu YL, Wang F, Li C, Dong YY, Huang ZX (2012) Novel low-temperature-active, salt-tolerant and proteases-resistant endo-1,4-β-mannanase from a new Sphingomonas strain. J Biosci Bioeng 113:568–574
Zhou JP, Peng MZ, Zhang R, Li JJ, Tang XH, Xu B, Ding JM, Gao YJ, Ren JR, Huang ZX (2015a) Characterization of Sphingomonas sp. JB13 exo-inulinase: a novel detergent-, salt-, and protease-tolerant exo-inulinase. Extremophiles 19:383–393
Zhou JP, Shen JD, Zhang R, Tang XH, Li JJ, Xu B, Ding JM, Gao YJ, Xu DY, Huang ZX (2015b) Molecular and biochemical characterization of a novel multidomain xylanase from Arthrobacter sp. GN16 isolated from the feces of Grus nigricollis. Appl Biochem Biotech 175:573–588
Zhou JP, Liu Y, Lu Q, Zhang R, Wu Q, Li CY, Li JJ, Tang XH, Xu B, Ding JM, Han NY, Huang ZX (2016a) Characterization of a glycoside hydrolase family 27 α-galactosidase from Pontibacter reveals its novel salt–protease tolerance and transglycosylation activity. J Agr Food Chem 64:2315–2324
Zhou JP, Lu Q, Zhang R, Wang YY, Wu Q, Li JJ, Tang XH, Xu B, Ding JM, Huang ZX (2016b) Characterization of two glycoside hydrolase family 36 α-galactosidases: novel transglycosylation activity, lead–zinc tolerance, alkaline and multiple pH optima, and low-temperature activity. Food Chem 194:156–166
Zhou JP, Song ZF, Zhang R, Ding LM, Wu Q, Li JJ, Tang XH, Xu B, Ding JM, Han NY, Huang ZX (2016c) Characterization of a NaCl-tolerant β-N-acetylglucosaminidase from Sphingobacterium sp. HWLB1. Extremophiles 20:547–557
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31260215, 31660445), the Yunling Scholars (Grant No. 2015 56), the Yunling Industry Leading Talents (Grant No. 2014 1782), the Reserve Talents Project for Young and Middle-Aged Academic and Technical Leaders of Yunnan Province (Grant No. 2015HB033), and the Applied and Basic Research Foundation of Yunnan Province (Grant No. 201401PC00224).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by A. Driessen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, J., Song, Z., Zhang, R. et al. A Shinella β-N-acetylglucosaminidase of glycoside hydrolase family 20 displays novel biochemical and molecular characteristics. Extremophiles 21, 699–709 (2017). https://doi.org/10.1007/s00792-017-0935-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-017-0935-1