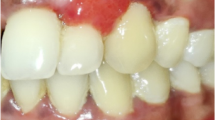
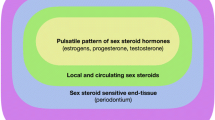
Abstract
Objectives
To investigate the oral manifestations in women of reproductive age using hormonal contraceptive methods.
Materials and methods
This review is based on the PRISMA statement. A literature search incorporated observational studies from the last 21 years. An investigative question was formulated using the PICO model, studies were selected, and a quality analysis was performed using the modified STROBE guidelines. A bibliometric analysis was performed, and the data were examined.
Results
Thirteen articles were included, with the majority evaluating periodontal status. Others analyzed factors such as the presence of alveolar osteitis, oral candidiasis, and salivary microbiome dysbiosis. Ten articles were deemed to have a low risk of bias.
Conclusions
Hormonal contraceptives may increase the risk of alveolar osteitis following tooth extraction and increase the presence of the Candida species in the oral cavity. They also affect the periodontium, such as the frequent development of gingivitis, but do not lead to changes in the salivary microbiome.
Clinical relevance
The increasing number of women using hormonal contraceptives and the knowledge that these contraceptives can produce oral cavity alterations underscore the need to evaluate the oral manifestations found in these women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout her life, a woman experiences hormonal fluctuations that can lead to bodily changes, having specific implications for her oral microflora [1, 2].
Hormonal contraceptives are based on synthetic combinations of estrogen and progesterone or progesterone alone, mimicking a state of pregnancy to prevent ovulation [3,4,5]. The mechanisms they use to hinder implantation are based on ovulation inhibition, alterations in the cervical mucus, and modifications in the endometrium [6, 7].
Progestin, which simulates endogenous progesterone and provides contraceptive action, is most commonly used [8, 9]. However, contraceptives that combine with estrogen, which participate in ovulation inhibition, regulate bleeding, and maintain endometrial thinning produced by progestin [8, 10] are also used.
In recent years, an increasing number of contraceptive methods have been developed, allowing women to choose the most suitable method based on their individual situation, needs, and preferences [11]. Additionally, these methods empower women to exert greater control over family planning, which is one of the most significant tasks of the special research program on Human Reproduction produced by the World Health Organization (WHO) [11, 12].
Combined oral contraceptives are among the most prescribed drugs worldwide [13,14,15]. Over time, they have undergone modifications to reduce their side effects [6, 8]. The first modification involved reducing the estrogenic component, and the second involved the development of new progestins to increase safety and limit androgenic side effects [6, 16]. The traditional way of administering oral contraceptives is in a 28-day cycle, but products with extended and continuous regimens have been introduced to reduce menstrual symptoms and the number of bleeding days. Furthermore, the hormone content in each pill can vary, with monophasic pills maintaining the exact dosage throughout the cycle, biphasic pills changing once, and triphasic pills changing twice [8, 17].
The suitable option for women with medical issues or side effects related to estrogens is the progestin-only pill, also known as the “Mini-Pill” [8, 16]. Progestin doses are low and result mainly in endometrial thinning, increased cervical mucus thickness, and reduced tubal motility. The dosage is continuous, without a hormone-free interval, and, to achieve good results, the pill must be taken every day [8, 18].
As an alternative, hormonal intrauterine devices (IUDs), which did not gain acceptance as a contraceptive method until the creation of Mirena® in 2001 [19] have become popular. This device has a T shape containing a reservoir with 52 mg of levonorgestrel and polydimethylsiloxane, which regulates its release [6]. As this method prevents fertilization, it is not considered an abortive method and is effective for at least 5 years [8].
New administration systems have recently been incorporated to improve tolerability, convenience, and compliance. These include injectable, transdermal, vaginal, and implantable systems [6, 20]. Emergency contraception, commonly known as the “morning-after pill,” contains 1.5 mg of levonorgestrel [16, 21] and is effective up to 72 h but should be taken as soon as possible after sexual intercourse. Its efficacy results from ovulation inhibition or delay, and its safety allows it to be sold without a prescription in many European countries [6, 16].
The use of hormonal contraceptives could lead to problems in the oral cavity, necessitating specific attention and care [22, 23]. The medical records obtained during a dental consultation should include the use of contraceptives, and women of reproductive age should be questioned about their use [24, 25]. The dentist must have up-to-date knowledge of these methods to properly advise patients and address any concerns that may arise [26, 27].
The increasing number of women using hormonal contraceptives and the awareness that these contraceptives can cause alterations in the oral cavity highlight the need for a systematic review synthesizing the oral manifestations that may occur in these women. Therefore, this study aimed to conduct a qualitative synthesis of studies to determine possible oral manifestations that may appear in women of reproductive age using hormonal contraceptive methods.
Materials and methods
This systematic review was conducted following the PRISMA 2020 guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [28] and was registered with PROSPERO (International prospective register of systematic reviews) under the identification number CRD42022378210 [29]. Additionally, the PICO model [30] was used to formulate the following research question: What are the oral manifestations present in women of reproductive age using hormonal contraceptives? (P: women of reproductive age using hormonal contraceptives; I: presence of oral manifestations; C: risk of oral manifestations in women of reproductive age using hormonal contraceptives compared with those not using hormonal contraception methods; and O: prevalence of oral manifestations in women of reproductive age using hormonal contraception methods).
The search strategy, study selection process, data extraction, and quality assessment (risk of bias assessment) were performed by two independent investigators (M.G.R. and J.G.G.). In case of doubt, a third investigator was consulted (MR.P.LL.).
Search strategy
The search was conducted in November 2023 across five electronic databases (MEDLINE, Web of Science, Scopus, Cochrane Library, and SciELO). In all databases, the search was limited to articles published between January 2002 and November 2023. The search strategy was established using the terms shown in Table 1, combined using the Boolean operators “OR” and “AND.” Additionally, advanced search symbols such as (*) were used for word truncation.
Inclusion and exclusion criteria
The inclusion and exclusion criteria are presented in Table 2 and were established based on the research question and study objectives.
Study selection
The bibliographic references obtained through the search strategy were exported to the citation manager EndNote (Clarivate Analytics, London, United Kingdom) to remove potential duplicates. A screening process was performed by reviewing the titles and, subsequently, the abstracts based on the inclusion and exclusion criteria. Next, the articles that met these criteria were assessed for eligibility and qualitative synthesis through a full-text screening.
Study data
For the bibliometric analysis, the following information was recorded for each article: author and year of publication, journal, and country of publication. Additionally, a table was created to summarize the following data: author and year, study design, study groups or sample, age of participants, type of hormonal contraceptive used, oral manifestations, outcomes of interest, and conclusions.
Quality analysis
To assess the risk of bias in the selected articles, a modified STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) analysis was used [31] (Table 3). This analysis consisted of 11 criteria derived from items 5, 6, 7, 8, 10, 12, 14, and 15 of the original STROBE checklist. During the evaluation, compliance with each criterion was indicated with a check mark (✓), while non-compliance was marked with a cross (×). Articles were categorized based on their scores: those with 8 to 11 points were considered as having a low risk, those with 4 to 7 points were considered as having a moderate risk, and those with 3 points or below were considered as having a high risk of bias.
Results
Study selection and flow diagram
The literature search yielded a total of 573 results. Specifically, 96 articles were retrieved from MEDLINE (PubMed), 290 from Web of Science, 140 from Scopus, 46 from Cochrane Library, and 1 from SciELO. The results obtained from each database are summarized in Table 4.
After discarding 119 duplicate articles, 454 were selected for title and abstract screening. Expressly, 399 articles were excluded after reviewing their titles, and an additional 55 articles were excluded after reading the abstracts and verifying that they did not meet the inclusion criteria. Next, the 17 remaining articles were assessed through full-text reading, and, ultimately, 13 articles were chosen for qualitative analysis (Fig. 1).
Characteristics of the studies
Bibliometric analysis
The organization of the selected articles based on the year of publication is presented in Fig. 2, country in Fig. 3, and journal in Fig. 4.
Study design
Among the selected studies for the review, the following study designs were found: 6 case-control studies [32,33,34,35,36,37], which represent 46.15% of the 13 chosen articles; 5 cross-sectional studies [38,39,40,41,42], also accounting for 38.46% of the total; and 1 cohort study [43] and 1 longitudinal study [44], each representing 7.69% of the total (Table 5).
Groups or sample
The sample size was quite variable among the articles (Table 5), with sample sizes below 100 participants [32, 33, 35,36,37, 42], while others exceeded this value [34, 38,39,40,41, 43, 44]. Additionally, two studies [38, 44] did not specify the exact number of women using contraceptives in the total sample. It is worth noting the presence of two articles [40, 41] with sample sizes of n = 9931 and n = 4460, respectively. These articles achieved representative population samples by using data from individuals surveyed in previous years.
Age of participants
To indicate the age of the participants, 8 articles provided the mean age of the studied groups, representing 61.53% of the total [32, 33, 36, 37, 39, 40, 42, 43] as shown in Table 5. However, 4 articles provided an age range accounting for 30.76% [34, 35, 38, 41] of the total. One study [44] representing 7.69% of the selected articles included women using a contraceptive method but did not provide any age data,.
Type of hormonal contraceptive
The majority of studies include patients using oral contraceptives [32,33,34,35,36,37,38,39,40, 42,43,44], accounting for 92.30% of all selected articles. Within this group, 7 articles [32,33,34,35, 40, 42, 43] specified that the included oral contraceptives were based on combined methods. Despite including a group of women using oral contraceptives, one study [43] also evaluated another set of women using the hormonal levonorgestrel intrauterine device (IUD) as a contraceptive method. One article [41] exclusively studied a group of women using a non-oral contraceptive method, the injectable medroxyprogesterone acetate contraceptive (Table 5).
Oral manifestations
Among the oral manifestations listed in Table 5, those related to the periodontal status of the patients were the most prevalent and were studied in 10 articles [32,33,34, 36,37,38,39,40,41,42], accounting for 76.92% of the selected articles. Within this group, one of the articles also studied the subgingival presence of specific periodontopathogens [32], and others [36] explored various oral manifestations, such as reduced orthodontic tooth movement, ulcerative lesions, changes in mucosal color, and the presence of pyogenic granuloma. Another article [37] also studies the presence of gingival inflammation, changes in mucosal color, pyogenic granuloma and oral ulcers, but additionally measures changes in salivary flow, pH, and biochemical data such as total salivary protein, alkaline phosphatase, and immunoglobulin A (Ig A). However, some articles investigated other types of oral alterations, such as oral candidiasis [35], dysbiosis in the salivary microbiome [43], or alveolar osteitis after extraction [44].
Quality analysis
The quality analysis used for this systematic review was based on a modified version of the STROBE guidelines for observational studies [31]. Ten studies were considered to have a low risk (76.92%), one study had moderate risk (7.69%), and two studies had high risk (15.38%) of bias. The studies classified as high risk presented only 3 of the evaluated criteria [36, 37]. The study classified as moderate risk [33] obtained a final score of 7. Among the studies with the lowest risk of bias, only one article [40] met all 11 criteria, while the rest met 10 [41, 43], 9 [32, 35, 44], and 8 [34, 38, 39, 42] criteria, respectively (Table 6).
Discussion
This systematic review aimed to discover the association of hormonal contraceptives with oral manifestations. Regarding the results, it is essential to highlight the presence of two high-risk articles [36, 37]. The low reliability obtained from the quality analysis justifies the decision to discard its data.
It has been reported that women who take oral contraceptives have a higher risk of experiencing alveolar osteitis after a dental extraction because estrogen causes a variation in coagulation and fibrinolytic factors, leading to a more significant dissolution of clots and hindering proper healing [3]. Parthasarathi et al. [44] examined a group of individuals who underwent extractions. They reported that none of the patients taking contraceptives subsequently developed alveolar osteitis. However, meta-analyses, including by Bienek et al., [45] determined that the use of oral contraceptives almost doubles the risk of developing alveolar osteitis, while Tang et al. [46] indicated that a relationship may exist between hormonal dosage and the incidence of alveolar osteitis.
Aminzadeh et al. [35] attempted to link the use of combined oral contraceptives with oral candidiasis. Estrogen has been reported to enhance the growth and adhesion of the Candida species to vaginal epithelial cells [47], and Aminzadeh et al. [35] investigated the potential colonization of these species in the oral cavity of women who take oral contraceptives containing estrogen. They concluded that oral contraceptives may increase the colonization of C. albicans and C. krusei, but their use did not determine the development of the disease in these women. Furthermore, they recommended conducting further research to link the use of oral contraceptives to the adherence of the Candida species in the oral epithelium, similar to what other authors, including Gonçalves et al. [48], have recommended regarding vaginal candidiasis.
Steroid hormones can indirectly lead to changes in periodontal tissue [13, 49], and the presence of estrogen and progesterone receptors has been demonstrated in the gingivae. Estrogen receptors are present in periosteal fibroblasts, fibroblasts scattered in the lamina propria, and fibroblasts and osteoblasts of the periodontal ligament [3, 13]. These receptors bind to specific hormones, which accumulate and are retained within the tissues and can bring about changes in gingival response [13]. Mullally et al. [42] reported that women using combined oral contraceptives had poorer periodontal health. Studies, including the research conducted by Domingues et al. [50], indicate that combined oral contraceptives can impact the periodontal condition in women, potentially leading to heightened gingival inflammation.Additionally, Mullally et al. [42] determined that, among women with aggressive forms of periodontal disease such as generalized aggressive periodontitis, the current or prior use of combined oral contraceptives was common. Therefore, they reported that medication, even in formulations with less estrogen, promoted increased periodontal destruction in patients susceptible to the disease. However, Taichman and Eklund [40] concluded that low-dose and high-dose combined oral contraceptives were not associated with the increased occurrence of gingivitis or periodontitis but were unable to determine a protective effect from them. Their study presented a representative sample of the population, as they obtained information through national health and nutrition examination surveys (NHANES). The self-selection of data to make the surveys comparable and the use of different methods to evaluate the presence or absence of periodontal disease may have influenced the study’s outcome. Perhaps contraceptives might not have adverse effects on most women’s periodontal health, but they could be considered a risk for those susceptible to aggressive forms of periodontal disease [42].
Brusca et al. [32] supported Mullaly et al.‘s [42] theory that female users of combined oral contraceptives had worse periodontal health due to higher and significant levels of severe periodontitis. Moreover, Brusca et al. [32] determined that these patients presented a higher number of specific periodontal pathogens in periodontal pockets, including Porphyromonas gingivalis, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, and a wide variety of Candida species. Specifically, C. albicans, C. parapsilosis, C. krusei, C. tropicalis, and C. glabrata were the species capable of surviving after 3 years of hormonal therapy, while C. dubliniensis was not isolated in the periodontal pockets. A more recent study by Arumugam et al. [51] confirmed the influence of oral or injectable contraceptives on the occurrence of Candida species in periodontal pockets. However, both studies reported that the association between Candida and periodontitis was controversial.
Haerian-Ardakani et al. [33] studied a group consisting of women taking low-dose combined oral contraceptives for at least 2 years. Their findings were consistent with those of Mullally et al. [42] in that these women showed more bleeding and the presence of gingivitis.
Wu et al. [38] and Smadi and Zakaryia [34] also determined that the use of oral contraceptives exacerbated gingival inflammation and the development of gingivitis. These results are consistent with previous studies such as the one by Tilakaratne et al. [52], which argue that hormonal contraceptives are associated with a higher prevalence of gingivitis.
Furthermore, Smadi and Zakaryia [34] indicated that androgens played a relevant role in maintaining bone mass and could suppress osteoclastic functions or the synthesis of prostaglandins and interleukin 6 (IL-6) during an inflammatory process. They also stated that recently introduced oral contraceptives, in addition to lower estrogen doses, exhibited changes in the progesterone component to induce fewer androgenic side effects. The fact that the effects on the periodontium remained evident led them to conclude that gingival disease was exacerbated by the use of new generations of combined oral contraceptives, which lacked the potential androgenic protective effect. This study highlighted the need for research that examines the effect of each type of progesterone on oral health, including those in contraception methods using different administration routes.
Prachi et al. [39], based on the community periodontal index and the index of loss of attachment, determined that women taking oral contraceptives had worse periodontal and gingival health. Their results were consistent with those of Mullally et al. [42]. Furthermore, Prachi et al. [39] reported a significant association between these indices and the duration of contraceptive therapy. A review by Ali et al. [53] determined that changes in the periodontium appeared after a few months and gradually increased with the duration of therapy.
Taichman et al. [41] investigated the relationship between periodontal diseases and the injectable method of medroxyprogesterone acetate (DMPA). They observed a significant association between the current use of DMPA and gingivitis and a modest association with periodontitis. The study by Bagheri et al. also supports that the use of DMPA has effects on the periodontal health of patients [54].
However, in Taichman et al.‘s study, [41] it was puzzling that female smokers using DMPA had a lower likelihood of periodontal disease. This could lead to speculation that both factors do not synergistically increase the risk of periodontitis, but further studies are needed to clarify this finding. Similar to Taichman and Eklund [40], this data is cross-sectional and based on NHANES surveys. Authors like Eke et al. [55] criticize that the periodontal examinations they use underestimate the prevalence of periodontitis, leading to inaccurate results in disease classification.
Finally, Bostanci et al. [43] studied the influence of hormonal fluctuations on the dysbiosis or imbalance of the salivary microbiome. The study reported that hormonal contraceptives were not associated with significant changes in the salivary microbiome. These findings were consistent with those of Krog et al., [56] who reported that hormonal contraceptives used by healthy young women did not have a significant relationship with the composition of the salivary microbiome.
Limitations of the study included that articles that did not include the terms used in the search strategy, whether related to hormonal contraception or the oral cavity, were not evaluated. In addition, the search, to select articles effectively, was limited to the past 20 years due to low search result figures. To the authors’ knowledge, this was the first systematic review that evaluated the different oral manifestations that may appear in women using any form of hormonal contraception. New research that avoids variability in study execution and considers potential modifying factors such as the type of hormonal compound, hormonal dosage, therapy duration, or tobacco use is essential to evaluate the oral manifestations produced by various types of hormonal contraceptives. This approach will enable precise comparisons to establish robust conclusions about the topic. By investigating whether such hormones increase the risk of oral manifestations, we can present comparative data between women undergoing therapy and those not in treatment. Therefore, establishing a single experimental protocol is necessary to facilitate the interpretation of the data obtained. This will allow us to extrapolate our results to the general population It is crucial to note that while a meta-analysis would ideally offer a quantitative comparison of the effects of hormonal contraceptive methods on oral manifestations, conducting such an analysis is not feasible within the current scope of our research. The limited availability of specific studies and the heterogeneity of the reported data prevent us from performing a meta-analysis with the necessary statistical rigor. This limitation underscores the need for further research in this area to accumulate a more extensive and homogeneous data set, enabling future meta-analytical evaluations.
Conclusions
Based on the results, we determined that hormonal contraceptives may increase the risk of alveolar osteitis after tooth extraction and promote the presence of the Candida species in the oral cavity. However, no evidence of their association with the development of oral candidiasis was found. Additionally, hormonal contraceptives affect the periodontium, including increased gingival inflammation, worsened development of periodontitis in susceptible patients, and a higher presence of specific periodontopathogens in periodontal pockets. Hormonal contraceptives, however, are not associated with significant changes in the salivary microbiome.
References
Boyapati R, Cherukuri SA, Bodduru R, Kiranmaye A (2021) Influence of female sex hormones in different stages of women on Periodontium. J Midlife Health 12(4):263–266. https://doi.org/10.4103/jmh.jmh_142_21
Burakoff RP (2003) Preventive dentistry: current concepts in women’s oral health. Prim Care Update OB/GYNS 10(3):141–146. https://doi.org/10.1016/s1068-607x(03)00021-0
Guncu GN, Tozum TF, Caglayan F (2005) Effects of endogenous sex hormones on the periodontium review of literature. Aust Dent J 50(3):138–145. https://doi.org/10.1111/j.1834-7819.2005.tb00352.x
Mascarenhas P, Gapski R, Al-Shammari K, Wang HL (2003) Influence of sex hormones on the periodontium. J Clin Periodontol 30(8):671–681. https://doi.org/10.1034/j.1600-051X.2003.00055.x
Hughes FJ, Bartold PM (2018) Periodontal complications of prescription and recreational drugs. Periodontol 2000 78(1):47–58. https://doi.org/10.1111/prd.12230
Hormonal contraception (2008) Recent advances and controversies. Fertil Steril 90(5 Suppl):S103–S113. https://doi.org/10.1016/j.fertnstert.2008.08.093
Rivera R, Yacobson I, Grimes D (1999) The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol 181(5 Pt 1):1263–1269. https://doi.org/10.1016/s0002-9378(99)70120-1
Blumenthal PD, Edelman A (2008) Hormonal contraception. Obstet Gynecol 112(3):670–684. https://doi.org/10.1097/AOG.0b013e31818425b7
Edwards M, Can AS (2022) Progestin. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022. StatPearls Publishing LLC.
Fruzzetti F, Fidecicchi T, Montt Guevara MM, Simoncini T (2021) Estetrol: a New Choice for Contraception. J Clin Med 10(23). https://doi.org/10.3390/jcm10235625
Both S, Lew-Starowicz M, Luria M, Sartorius G, Maseroli E, Tripodi F et al (2019) Hormonal contraception and female sexuality: position statements from the European Society of Sexual Medicine (ESSM). J Sex Med 16(11):1681–1695. https://doi.org/10.1016/j.jsxm.2019.08.005
Carbajal-Ugarte JA, Cardenas-Blanco A, Pastrana-Huanaco E, Lopez-Berrios D (2008) [Efficacy and adverse effects of hormonal contraceptives: comparative study]. Rev Med Inst Mex Seguro Soc 46(1):83–87
Sathish AK, Varghese J, Fernandes AJ (2022) The impact of sex hormones on the Periodontium during a woman’s lifetime: a concise-review update. Curr Oral Health Rep 9(4):146–156. https://doi.org/10.1007/s40496-022-00321-0
Jeske AH (2019) Endocrine drugs of significance in Dentistry. Contemporary Dental Pharmacology: evidence-based considerations. Springer International Publishing, pp 85–90
Heasman PA, Hughes FJ (2014) Drugs, medications and periodontal disease. Br Dent J 217(8):411–419. https://doi.org/10.1038/sj.bdj.2014.905
Erkkola R (2007) Recent advances in hormonal contraception. Curr Opin Obstet Gynecol 19(6):547–553. https://doi.org/10.1097/GCO.0b013e3282f1e7b6
Nappi RE, Kaunitz AM, Bitzer J (2016) Extended regimen combined oral contraception: a review of evolving concepts and acceptance by women and clinicians. Eur J Contracept Reprod Health Care 21(2):106–115. https://doi.org/10.3109/13625187.2015.1107894
Hee L, Kettner LO, Vejtorp M (2012) Continuous use of oral contraceptives: an overview of effects and side-effects. Acta Obstet Gynecol Scand 92(2):125–136. https://doi.org/10.1111/aogs.12036
Hsia JK, Creinin MD (2016) Intrauterine contraception. Semin Reprod Med 34(3):175–182. https://doi.org/10.1055/s-0036-1571438
Bahamondes L, Bahamondes MV (2014) New and emerging contraceptives: a state-of-the-art review. Int J Women’s Health. https://doi.org/10.2147/ijwh.S46811
Weismiller DG (2004) Emergency contraception. Am Fam Physician 70(4):707–714
Kessler JL, A Literature Review on Women’s Oral Health Across the Life Span (2017) Nurs Womens Health 21(2):108–121. https://doi.org/10.1016/j.nwh.2017.02.010
Bal SCB, Oberoi SS, Dalai RP, Sethy S (2020) Hormonal changes across the life cycle of women and its effects on the periodontium. Indian J Forensic Med Toxicol 14(4):8258–8263. https://doi.org/10.37506/ijfmt.v14i4.12975
Otomo-Corgel J (2013) Dental management of the female patient. Periodontol 2000 61:219–231. https://doi.org/10.1111/j.1600-0757.2011.00411.x
How medications can affect your oral health (2005) J Am Dent Assoc 136(6):831. https://doi.org/10.14219/jada.archive.2005.0269
Preshaw P PM (2013) Oral contraceptives and the periodontium. Periodontol 2000 61(1):125–159. https://doi.org/10.1111/j.1600-0757.2011.00399.x
Kishore M, Panat SR, Aggarwal A, Agarwal N, Upadhyay N, Alok A (2014) Evidence based dental care: integrating clinical expertise with systematic research. J Clin Diagn Res 8(2):259–262. https://doi.org/10.7860/JCDR/2014/6595.4076
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Schiavo JH (2019) PROSPERO: An International Register of systematic review protocols. Med Ref Serv Q 38(2):171–180. https://doi.org/10.1080/02763869.2019.1588072
da Costa Santos CM, de Mattos Pimenta CA, Nobre MR (2007) The PICO strategy for the research question construction and evidence search. Rev Lat Am Enfermagem 15(3):508–511. https://doi.org/10.1590/s0104-11692007000300023
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2008) The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61(4):344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008
Brusca MI, Rosa A, Albaina O, Moragues MD, Verdugo F, Ponton J (2010) The impact of oral contraceptives on women’s Periodontal Health and the Subgingival occurrence of aggressive periodontopathogens and Candida Species. J Periodontol 81(7):1010–1018. https://doi.org/10.1902/jop.2010.090575
Haerian-Ardakani A, Moeintaghavi A, Talebi-Ardakani MR, Sohrabi K, Bahmani S, Dargahi M (2010) The association between current low-dose oral contraceptive pills and periodontal health: a matched-case-control study. J Contemp Dent Pract 11(3):033–40
Smadi L, Zakaryia A (2018) The association between the use of new oral contraceptive pills and periodontal health: a matched case-control study. J Int Oral Health 10(3):127–131. https://doi.org/10.4103/jioh.jioh_17_18
Aminzadeh A, Sabeti Sanat A, Nik Akhtar S (2016) Frequency of Candidiasis and colonization of Candida albicans in relation to oral contraceptive pills. Iran Red Crescent Med J 18(10):e38909. https://doi.org/10.5812/ircmj.38909
Altaee ZH (2020) Effect of contraceptive pills in tooth movement and oral manifestation in orthodontic patients. Int Med J 27(2):209–211
Rasheed RH, Faisal Ahmed R (2023) The effects of oral birth control pills (contraceptive pills) intake on lyophilized saliva and its oral manifestations. Bionatura 8(1):1–5. https://doi.org/10.21931/rb/2023.08.01.71
Wu YM, Liu J, Sun WL, Chen LL, Chai LG, Xiao X et al (2013) Periodontal status and associated risk factors among childbearing age women in Cixi City of China. J Zhejiang Univ Sci B 14(3):231–239. https://doi.org/10.1631/jzus.B1200034
Prachi S, Jitender S, Rahul C, Jitendra K, Priyanka M, Disha S (2019) Impact of oral contraceptives on periodontal health. Afr Health Sci 19(1):1795–1800. https://doi.org/10.4314/ahs.v19i1.56
Taichman LS, Eklund SA (2005) Oral contraceptives and periodontal diseases: rethinking the association based upon analysis of National Health and Nutrition Examination Survey data. J Periodontol 76(8):1374–1385. https://doi.org/10.1902/jop.2005.76.8.1374
Taichman LS, Sohn W, Kolenic G, Sowers M (2012) Depot medroxyprogesterone acetate use and periodontal health in 15- to 44-year-old US females. J Periodontol 83(8):1008–1017. https://doi.org/10.1902/jop.2012.110534
Mullally BH, Coulter WA, Hutchinson JD, Clarke HA (2007) Current oral contraceptive status and periodontitis in young adults. J Periodontol 78(6):1031–1036. https://doi.org/10.1902/jop.2007.060163
Bostanci N, Krog MC, Hugerth LW, Bashir Z, Fransson E, Boulund F et al (2021) Dysbiosis of the human oral Microbiome during the Menstrual cycle and vulnerability to the External exposures of Smoking and Dietary Sugar. Front Cell Infect Microbiol 11:625229. https://doi.org/10.3389/fcimb.2021.625229
Parthasarathi K, Smith A, Chandu A (2011) Factors affecting incidence of dry socket: a prospective community-based study. J Oral Maxillofac Surg 69(7):1880–1884. https://doi.org/10.1016/j.joms.2010.11.006
Bienek DR, Filliben JJ (2016) Risk assessment and sensitivity meta-analysis of alveolar osteitis occurrence in oral contraceptive users. J Am Dent Assoc 147(6):394–404. https://doi.org/10.1016/j.adaj.2016.01.011
Tang M, Gurpegui Abud D, Shariff JA (2022) Oral contraceptive use and alveolar Osteitis following third molar extraction: a systematic review and Meta-analysis. Int J Dent 2022:7357845. https://doi.org/10.1155/2022/7357845
Fidel PL Jr., Cutright J, Steele C (2000) Effects of reproductive hormones on experimental vaginal candidiasis. Infect Immun 68(2):651–657. https://doi.org/10.1128/iai.68.2.651-657.2000
Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S (2016) Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit Rev Microbiol 42(6):905–927. https://doi.org/10.3109/1040841x.2015.1091805
Castro MML, Ferreira MKM, Prazeres IEE, de Oliveira Nunes PB, Magno MB, Rösing CK et al (2021) Is the use of contraceptives associated with periodontal diseases? A systematic review and meta-analyses. BMC Womens Health 21(1):48. https://doi.org/10.1186/s12905-021-01180-0
Domingues RS, Ferraz BF, Greghi SL, Rezende ML, Passanezi E, Sant’Ana AC (2012) Influence of combined oral contraceptives on the periodontal condition. J Appl Oral Sci 20(2):253–259. https://doi.org/10.1590/s1678-77572012000200022
Arumugam M, Seshan H (2015) A comparative evaluation of subgingival occurrence of Candida species in periodontal pockets of female patients using hormonal contraceptives and non-users–A clinical and microbiological study. Oral Health Dent Manag 14(4):206–211
Tilakaratne A, Soory M, Ranasinghe AW, Corea SM, Ekanayake SL, de Silva M (2000) Effects of hormonal contraceptives on the periodontium, in a population of rural Sri-Lankan women. J Clin Periodontol 27(10):753–757. https://doi.org/10.1034/j.1600-051x.2000.027010753.x
Ali I, Patthi B, Singla A, Gupta R, Dhama K, Niraj LK et al (2016) Oral health and oral contraceptive - is it a Shadow behind Broad Day light? A systematic review. J Clin Diagn Res 10(11):Ze01–ze6. https://doi.org/10.7860/jcdr/2016/19439.8790
Bagheri F, Tadayon M, Afshari P, Jahangirneghad M, Haghighizadeh MH (2016) Association between Depot-Medroxyprogesterone Acetate Injection and Periodontal Health in Reproductive Age women: a Case Control Study in Iran. Jundishapur J Chronic Disease Care 5(3). https://doi.org/10.17795/jjcdc-36922
Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA (2010) Accuracy of NHANES periodontal examination protocols. J Dent Res 89(11):1208–1213. https://doi.org/10.1177/0022034510377793
Krog MC, Hugerth LW, Fransson E, Bashir Z, Nyboe Andersen A, Edfeldt G et al (2022) The healthy female microbiome across body sites: effect of hormonal contraceptives and the menstrual cycle. Hum Reprod 37(7):1525–1543. https://doi.org/10.1093/humrep/deac094
Funding
No funding was obtained for this study.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
The conception and design of the study, or acquisition of data, or analysis and interpretation of data M.G.R., M.R.P.LL., and J.G.G; drafting the article or revising it critically for important intellectual content, M.G.R., and J.G.G.; final approval of the version to be submitted M.G.R, M.R.P,LL., and J.G.G.
Corresponding author
Ethics declarations
Ethics aproval and consent to participate
Not applicable.
Conflict of interest
C. M. Garcia-Rojo declares that she has no conflict of interest. MR. Pecci-Lloret declares that he has no conflict of interest. J. Guerrero-Gironés declares that she has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rojo, M.G., Lloret, M. & Gironés, J.G. Oral manifestations in women using hormonal contraceptive methods: a systematic review. Clin Oral Invest 28, 184 (2024). https://doi.org/10.1007/s00784-024-05573-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05573-x