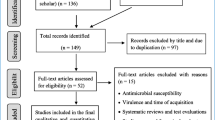
Abstract
Objective
This meta-analysis was designed to provide new insights into the relationship between Helicobacter pylori (H. pylori) infection and recurrent aphthous stomatitis (RAS).
Materials and methods
We included and evaluated studies on H. pylori infection and RAS from PubMed, EMBASE, Cochrane Library, and Web of Science databases published up to January 31, 2023. The characteristics of these studies were collected, and the quality was evaluated by Newcastle-Ottawa Scale (NOS). The random effects model was used to calculate the pooled odds ratio (OR) and 95% confidence interval (CI). To further explore the sources of heterogeneity, meta-regression analysis and subgroup analyses were performed. Funnel plot, Egger’s test, and Begg’s test were used to assess publication bias.
Results
In total, fifteen case-control studies with 1137 individuals (601 cases and 536 controls) were included. The H. pylori was found to be significantly associated with RAS (OR: 1.83 95% CI: 1.41–2.37, P = 0.001). In the subgroup analyses, studies that used PCR (OR: 2.03 95% CI: 1.31–3.15) or UBT (OR: 1.83 95% CI: 1.13–2.96) yielded a significant positive association, while a non-significant association (OR: 1.12 95% CI: 0.61–2.08) was found from studies that used ELISA method. Sensitivity analyses showed that the results were robust. No significant publication bias was found.
Conclusions
The current evidence does not rule out an association between H. pylori and RAS. The effect of H. pylori on RAS varies in detection methods and sources of sample. Large samples, multiple clinical studies, and improved methods are still needed to determine the exact effect of H. pylori on RAS.
Clinical significance
H. pylori infection may be a risk factor for the pathogenesis of RAS.



Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.
References
Rivera C, Muñoz-Pastén M, Núñez-Muñoz E, Hernández-Olivos R (2022) Recurrent aphthous stomatitis affects quality of life. A case-control study. Clin Cosmet Investig Dent 14:217–223. https://doi.org/10.1101/2022.04.01.22273300
Xu KY, Zhou CC, Huang F, Duan N, Wang YY, Zheng LC, Wang X, Wang WM (2021) Relationship between dietary factors and recurrent aphthous stomatitis in China: a cross-sectional study. J Int Med Res 5:30006052110177. https://doi.org/10.1177/03000605211017724
Rees TD, Binnie WH (1996) Recurrent aphthous stomatitis. Dermatol Clin 2:243–256. https://doi.org/10.1016/s0733-8635(05)70353-3
Wei X, Zhao HQ, Ma C, Zhang AB, Feng H, Zhang D, Liu C (2019) The association between chronic periodontitis and oral Helicobacter pylori: a meta-analysis. PLoS One 12:e0225247. https://doi.org/10.1371/journal.pone.0225247
Xue Y, Zhou LY, Lu HP, Liu JZ (2019) Recurrence of Helicobacter pylori infection: incidence and influential factors. Chin Med J 7:765–771. https://doi.org/10.1097/CM9.0000000000000146
Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC (2017) Global prevalence of Helicobacter pylori infection: systematic review and meta-Analysis. Gastroenterol 2:420–429. https://doi.org/10.1053/j.gastro.2017.04.022
Eusebi LH, Zagari RM, Bazzoli F (2014) Epidemiology of Helicobacter pylori infection. Helicobacter 1:1–5. https://doi.org/10.1111/hel.12165
Liu Y, Li R, Xue X, Xu T, Luo Y, Dong Q, Liu J, Liu J, Pan Y, Zhang D (2020) Periodontal disease and Helicobacter pylori infection in oral cavity: a meta-analysis of 2727 participants mainly based on Asian studies. Clin Oral Investig 7:2175–2188. https://doi.org/10.1007/s00784-020-03330-4
Li S, Zhang Y, Yang Z, Li J, Li Y, Li H, Li W, Jia J, Ge S, Sun Y (2021) Helicobacter pylori infection is correlated with the incidence of erosive oral lichen planus and the alteration of the oral microbiome composition. BMC Microbiol 1:122. https://doi.org/10.1186/s12866-021-02188-0
Gupta AA, Kheur S, Raj AT, Mahajan P (2020) Association of Helicobacter pylori with oral potentially malignant disorders and oral squamous cell carcinoma-a systematic review and meta-analysis. Clin Oral Investig 1:13–23. https://doi.org/10.1007/s00784-019-03125-2
Albanidou-Farmaki E, Giannoulis L, Markopoulos A, Fotiades S, Aggouridaki X, Farmakis K, Papanayotou P (2005) Outcome following treatment for Helicobacter pylori in patients with recurrent aphthous stomatitis. Oral Dis 1:22–26. https://doi.org/10.1111/j.1601-0825.2004.01053.x
Li L, Gu H, Zhang G (2014) Association between recurrent aphthous stomatitis and Helicobacter pylori infection: a meta-analysis. Clin. Oral Investig 6:1553–1560. https://doi.org/10.1007/s00784-014-1230-5
Gomes CC, Gomez RS, Zina LG, Amaral FR (2016) Recurrent aphthous stomatitis and Helicobacter pylori. Med Oral Patol Oral Cir Bucal 2:e187–e191. https://doi.org/10.4317/medoral.20872
Zou LT, Hu YL (2013) A meta-analysis of the association between Helicobacter pylori and recurrent oral ulcers. Infect Inflamm Repair 2:102–106. https://doi.org/10.3969/j.issn.1672-8521.2013.02.013
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 10:1006–1012. https://doi.org/10.1371/journal.pmed.1000097
Slebioda Z, Szponar E, Kowalska A (2014) Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp 3:205–215. https://doi.org/10.1007/s00005-013-0261-y
Wells GA, Wells G, Shea B, Shea B, O'Connell D, Peterson J, Welch, Losos M, Tugwell P, Ga SW, Zello GA, Petersen JA (2014) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Semantic Scholar https://www.semanticscholar.org/paper/The-Newcastle-Ottawa-Scale-(NOS)-for-Assessing-the-Wells-Wells/c293fb316b6176154c3fdbb8340a107d9c8c82bf. Accessed 13 Apr 2013
Stang A, Jonas S, Poole C (2018) Case study in major quotation errors: a critical commentary on the Newcastle–Ottawa scale. Eur J Epidemiol 11:1025–1031. https://doi.org/10.1007/s10654-018-0443-3
Mohr SB, Gorham ED, Alcaraz JE, Kane CJ, Macera CA, Parsons JK, Wingard DL, Garland CF (2011) Serum 25-hydroxyvitamin D and prevention of breast cancer: pooled analysis. Anticancer Res Sep 31(9):2939–48. https://pubmed.ncbi.nlm.nih.gov/21868542
Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A (2007) Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 1:53–77. https://doi.org/10.1002/sim.2528
Barili F, Parolari A, Kappetein PA, Freemantle N (2018) Statistical primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg 3:317–321. https://doi.org/10.1093/icvts/ivy163
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 4:1088–1101. https://doi.org/10.2307/2533446
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 7109:629–634. https://doi.org/10.1136/bmj.315.7109.629
Silva DG, Tinoco EM, Rocha GA, Rocha AM, Guerra JB, Saraiva IE, Queiroz DM (2010) Helicobacter pylori transiently in the mouth may participate in the transmission of infection. Mem Inst Oswaldo Cruz 5:657–660. https://doi.org/10.1590/s0074-02762010000500009
Long BJ, Chen K, Wu BL, Duan JM (2007) Detection of Helicobacter pylori in patients with recurrent oral ulcer. J Southern Med Univ 4:477–478. https://doi.org/10.3321/j.issn:1673-4254.2007.04.040
Al-Amad SH (2020) Helicobacter pylori and gastric hyperacidity, and their association with recurrent aphthous stomatitis. Int J Oral Maxillofac Surg 12:1599–1604. https://doi.org/10.1016/j.ijom.2020.05.013
Gülseren D, Karaduman A, Kutsal D, Nohutcu RM (2016) The relationship between recurrent aphthous stomatitis, and periodontal disease and Helicobacter pylori infection. Clin Oral Investig 8:2055–2060. https://doi.org/10.1007/s00784-015-1704-0
Rajendra K, Purnachandra SM, Patel PC, Cota J, Singh VR, Vatsal A (2017) A clinical and microbiological evaluation of Helicobacter pylori in recurrent aphthous stomatitis. J Contemp Dent Pract 12:1194–1197. https://doi.org/10.5005/jp-journals-10024-2199
Maleki Z, Sayyari AA, Alavi K, Sayyari L, Baharvand M (2009) A study of the relationship between Helicobacter pylori and recurrent aphthous stomatitis using a urea breath test. J Contemp Dent Pract 1:9–16. https://doi.org/10.5005/jcdp-10-1-9
Iamaroon A, Chaimano S, Linpisarn S, Pongsiriwet S, Phornphutkul K (2003) Detection of Helicobacter pylori in recurrent aphthous ulceration by nested PCR. J Oral Sci 2:107–110. https://doi.org/10.2334/josnusd.45.107
Victória JM, Kalapothakis E, Silva Jde F, Gomez RS (2003) Helicobacter pylori DNA in recurrent aphthous stomatitis. J Oral Pathol Med 4:219–223. https://doi.org/10.1034/j.1600-0714.2003.00136.x
Riggio MP, Lennon A, Wray D (2000) Detection of Helicobacter pylori DNA in recurrent aphthous stomatitis tissue by PCR. J Oral Pathol Med 10:507–513. https://doi.org/10.1034/j.1600-0714.2000.291005.x
Porter SR, Barker GR, Scully C, Macfarlane G, Bain L (1997) Serum IgG antibodies to Helicobacter pylori in patients with recurrent aphthous stomatitis and other oral disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 83:325–328. https://doi.org/10.1016/S1079-2104(97)90237-7
Saeed SA, Zaidan TF (2020) Clinical evaluation of recurrent aphthous stomatitis and its correlation with Helicobacter pylori. Indian J Forensic Med Toxicol 4:2154–2159. https://doi.org/10.37506/ijfmt.v14i4.11870
Uyar M, Kilic SB, Uyar Y, Ozkaya E, Iriz A, Eryilmaz A (2014) The place of the Helicobacter pylori on the etiopathogenesis of idiopathic recurrent aphthous stomatitis (RAS). ResearchGate. https://www.researchgate.net/publication/266208526. Accessed 5 August 2023
Elsheikh MN, Mahfouz ME (2005) Prevalence of Helicobacter pylori DNA in recurrent aphthous ulcerations in mucosa-associated lymphoid tissues of the pharynx. Arch Otolaryngol Head Neck Surg 9:804–808. https://doi.org/10.1001/archotol.131.9.804
Yakar T, Serin E, Coşar AM, Arslan Taş D, Ataç FB (2015) The relationship of recurrent aphthous stomatitis and Helicobacter pylori, cytokine gene polymorphism and cobalamin. Turk J Gastroenterol 4:1461–1464. https://doi.org/10.5152/tjg.2015.0161
Al-Ahmad A, Kürschner A, Weckesser S, Wittmer A, Rauberger H, Jakob T, Hellwig E, Kist M, Waidner B (2012) Is Helicobacter pylori resident or transient in the human oral cavity? J Med Microbiol 8:1146–1152. https://doi.org/10.1099/jmm.0.043893-0
Teoman I, Ozmeriç N, Ozcan G, Alaaddinoğlu E, Dumlu S, Akyön Y, Baloş K (2007) Comparison of different methods to detect Helicobacter pylori in the dental plaque of dyspeptic patients. Clin Oral Investig 3:201–205. https://doi.org/10.1007/s00784-007-0104-5
Allaker RP, Young KA, Hardie JM, Domizio P, Meadows NJ (2002) Prevalence of Helicobacter pylori at oral and gastrointestinal sites in children: evidence for possible oral-to-oral transmission. J Med Microbiol 4:312–317. https://doi.org/10.1099/0022-1317-51-4-312
Mansour-Ghanaei F, Asmar M, Bagherzadeh AH, Ekbataninezhad S (2005) Helicobacter pylori infection in oral lesions of patients with recurrent aphthous stomatitis. Med Sci Monit 11(12):CR576–CR579. https://pubmed.ncbi.nlm.nih.gov/16319788
Bodger K, Wyatt JI, Heatley RV (1997) Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumor necrosis factor-a secretion. Gut 6:739–744. https://doi.org/10.1136/gut.40.6.739
Gasbarrini A, Franceschi F, Arnuzzi A, Ojetti V, Candelli M, Torre ES, De Lorenzo A, Anti M, Pretolani S, Gasbarrini G (1999) Extradigestive manifestations of Helicobacter pylori infection. Gut Suppl 1:1–12. https://doi.org/10.1136/gut.45.2008.i9
Birek C, Grandhi R, McNeill K, Singer D, Ficarra G, Bowden G (1999) Detection of Helicobacter pylori in oral aphthous ulcers. J Oral Pathol Med 5:197–203. https://doi.org/10.1111/j.1600-0714.1999.tb02024.x
Diouf A, Martinez-Gomis J, Miquel M, Quesada M, Lario S, Sixou M (2009) Comparison of four different primer sets for detection of Helicobacter pylori in gastric biopsies and oral samples by using real-time PCR. Pathol Biol (Paris) 57(1):30–35. https://doi.org/10.1016/j.patbio.2008.07.008
Xue CY, Tan Y, Wang YJ (2020) Correlation analysis between oral Helicobacter pylori infection and peri-implantitis and its clinical indexes. J Huazhong University Sci Technol 6:728–731. https://doi.org/10.3870/j.issn.1672-0741.2020.06.015
Ye GQ (2012) Clinical detection of oral Helicobacter pylori infection: current hot spots in the field of diagnosis. Chin Med J 16:1084–1086. https://doi.org/10.3760/cma.j.issn.0376-2491.2012.16.002
Wang YK, Kuo FC, Liu CJ, Wu MC, Shih HY, Wang SS, Wu JY, Kuo CH, Huang YK, Wu DC (2015) Diagnosis of Helicobacter pylori infection: current options and developments. World J Gastroenterol 40:11221–11235. https://doi.org/10.3748/wjg.v21.i40.11221
Mao X, Jakubovics NS, Bächle M, Buchalla W, Hiller KA, Maisch T, Hellwig E, Kirschneck C, Gessner A, Al-Ahmad A, Cieplik F (2021) Colonization of Helicobacter pylori in the oral cavity - an endless controversy? Crit Rev Microbiol 5:612–629. https://doi.org/10.1080/1040841X.2021.1907740
Ding YF, Xiao XR, Li AM, Dai CB (2002) Detection of Helicobacter pylori infection in gastric and oral cavity. Mod Prev Med 4:474–475. https://doi.org/10.3969/j.issn.1003-8507.2002.04.004
Zhang QF, Yu XQ, Liu JT (2016) Research progress on detection methods of gastric Helicobacter pylori and oral Helicobacter pylori. Int J Gastroenterol 2:111–114. https://doi.org/10.3969/j.issn.1673-534X.2016.02.012
Deng XD, Qiang L, Chen X, Bao Y (2022) Research progress on detection technology of Helicobacter pylori. J Cancer Control Treat 7:659–665. https://doi.org/10.3969/j.issn.1674-0904.2022.07.013
Rasmussen LT, Labio RW, Gatti LL, Silva LC, Queiroz VF, Smith Mde A, Payão SL (2010) Helicobacter pylori detection in gastric biopsies, saliva and dental plaque of Brazilian dyspeptic patients. Mem Inst Oswaldo Cruz 3:326–330. https://doi.org/10.1590/s0074-02762010000300015
Assumpção MB, Martins LC, Melo Barbosa HP, Barile KA, de Almeida SS, Assumpção PP, Corvelo TC (2010) Helicobacter pylori in dental plaque and stomach of patients from Northern Brazil. World J Gastroenterol 24:3033–3039. https://doi.org/10.3748/wjg.v16.i24.3033
Cai H, Li W, Shu X, Peng K, Zhang Y, Jiang M (2014) Genetic variation of Helicobacter pylori in the oral cavity and stomach detected using thymine adenine cloning in children with chronic gastritis. Pediatr Infect Dis J 1:e1–e6. https://doi.org/10.1097/INF.0000000000000017
Acknowledgements
Thanks to James K. H. Tsoi, Hangzhou Qiantang Specialty Appointed Expert (Hospital of Stomatology, Zhejiang Chinese Medical University, Hangzhou), for his supervision and guidance on this article.
Funding
This work was supported by the Program of Xinmiao Talents in Zhejiang Province (grant no. 2022R41A044) and Innovation and Entrepreneurship Training Program for College Students of Zhejiang Province (grant no. S202210344118).
Author information
Authors and Affiliations
Contributions
Y. Chen: Study concept and Writing –review & editing, Supervision, Visualization. Y. Wu: Study design and Writing – review & editing. J. Shen: Conceptualization, Methodology, Formal analysis, Writing – original draft. Z. Ye: Methodology, Investigation, Formal analysis. H. Xie: Methodology, Investigation, Formal analysis. D. Ling: Conceptualization, Writing – review & editing, Supervision. The authors had full access to the data and take full responsibility for the results. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
For this type of study, formal consent is not required.
Competing interests
The authors declare no competing interests.
Registration
The review was registered in PROSPERO (ref: CRD42023404955).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 0.98 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, J., Ye, Z., Xie, H. et al. The relationship between Helicobacter pylori infection and recurrent aphthous stomatitis: a systematic review and meta-analysis. Clin Oral Invest 27, 6345–6356 (2023). https://doi.org/10.1007/s00784-023-05273-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05273-y