Abstract
Objectives
To assess whether in animals or patients with ≥ 1 tooth extracted, hyaluronic acid (HyA) application results in superior healing and/or improved complication management compared to any other treatment or no treatment.
Materials and methods
Three databases were searched until April 2022. The most relevant eligibility criteria were (1) local application of HyA as adjunct to tooth extraction or as treatment of alveolar osteitis, and (2) reporting of clinical, radiographic, histological, or patient-reported data. New bone formation and/or quality were considered main outcome parameters in preclinical studies, while pain, swelling, and trismus were defined as main outcome parameters in clinical studies.
Results
Five preclinical and 22 clinical studies (1062 patients at final evaluation) were included. In preclinical trials, HyA was applied into the extraction socket. Although a positive effect of HyA was seen in all individual studies on bone formation, this effect was not confirmed by meta-analysis. In clinical studies, HyA was applied into the extraction socket or used as spray or mouthwash. HyA application after non-surgical extraction of normally erupted teeth may have a positive effect on soft tissue healing. Based on meta-analyses, HyA application after surgical removal of lower third molars (LM3) resulted in significant reduction in pain perception 7 days postoperatively compared to either no additional wound manipulation or the application of a placebo/carrier. Early post-operative pain, trismus, and extent of swelling were unaffected.
Conclusions
HyA application may have a positive effect in pain reduction after LM3 removal, but not after extraction of normally erupted teeth.
Clinical relevance
HyA application may have a positive effect in pain reduction after surgical LM3 removal, but it does not seem to have any impact on other complications or after extraction of normally erupted teeth. Furthermore, it seems not to reduce post-extraction alveolar ridge modeling, even though preclinical studies show enhanced bone formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the healing process following tooth extraction is commonly uneventful, any subsequent pain may compromise patients’ well-being, while complications may also occur. For example, surgical extraction of semi-/fully impacted third molars is regularly associated with significant pain, swelling, and trismus [1,2,3], which are aggravated in case of development of alveolar osteitis (AO)—also called dry socket. AO is considered one of the most frequent complications of tooth extraction occurring in 20 to 35% of the cases of surgical extraction of lower third molars (LM3), and in 1.4 to 5% of (non-surgical) extraction of regularly erupted teeth [1, 2, 4]. Besides such early complications, which negatively affect patients’ quality of life, compromised extraction socket healing may also lead to significant hard tissue defects, either at the extraction site or at the neighboring teeth [5, 6]. For example, it has been reported that deep periodontal defects, e.g., probing pocket depths ≥ 7 mm, at the distal aspect of the second molar occur in almost every fourth patient after extraction of impacted LM3 [5].
To reduce patient morbidity and improve soft and hard tissue healing of extraction sockets, as well as for the treatment of early complications (e.g., AO), various materials and/or surgical techniques have been tested (e.g., application of collagen sponges, gels, blood derivates, various grafting materials) [7,8,9]. Increasing attention is recently put on hyaluronic acid (HyA), due to its anti-inflammatory and antibacterial properties [10,11,12] and its positive effects on soft and hard tissue healing. Specifically, preclinical studies have demonstrated a positive effect, histologically, on the healing of bone [13, 14] and periodontal defects [15, 16] after HyA application. Based on the results of the meta-analyses of a systematic review of clinical trials on surgical extraction of third molars, significantly reduced pain on the third and seventh postoperative day, but not on trismus, was reported in groups receiving HyA-based products [17]. In this context, a comprehensive assessment of the available preclinical and clinical evidence on the effect of HyA application in connection with tooth extraction in general, including the prevalence, extent, and/or management of complications is missing. Therefore, the present systematic review addressed the following PICOS (population (P), intervention (I), comparison (C), outcomes (O), and study design (S)) question: “In animals/patients having ≥ 1 tooth extracted, does application of HyA alone or combined with other products/carriers result in superior soft-/hard tissue healing, reduced morbidity, reduced complication rate, and/or improved complication management compared to any other treatment or no treatment?”.
Material and methods
Study protocol and study registration
The present work followed available guidelines for performing systematic reviews of preclinical [18] and clinical studies (Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA); Appendix 1) [19]. Both protocols were registered at the international prospective register of systematic reviews (PROSPERO), i.e., one for the preclinical (CRD42021266190) and one for the clinical trials (CRD42021266183).
Information sources, literature search, and eligibility criteria
The literature search was performed in 3 databases (i.e., Ovid (MEDLINE and CENTRAL), EMBASE, and Pubmed)) on October 14, 2021, and updated on April 7, 2022. Details on the search including the keywords are presented in Appendix 2. After removing the duplicates, titles and abstracts were screened for eligibility by 2 reviewers (DD, TL) and kappa values for the screened full texts and finally included publications were calculated. Any ambiguity was resolved in discussion with a third author (KB). Independent of the study type, studies were included if (a) written in English or German language, (b) the full text was available, and (c) clinical, radiographic, or histological data were provided. Additional inclusion criteria for the preclinical studies were (a) randomized and non-randomized controlled experiments, and (b) local application of a HyA-based product alone or in combination with another product in ≥ 1 of the groups after extraction of ≥ 1 tooth. Additional inclusion criteria for the clinical studies were (a) randomized controlled trial (RCT), controlled trial (CT), or case series with a minimum of 10 patients, and (b) local application of a HyA-based product alone or in combination with another product in ≥ 1 of the groups either after extraction of ≥ 1 tooth or as treatment of AO of ≥ 1 tooth.
Data collection and extraction
Two authors (DD, KB) independently extracted the data twice and any disagreement was resolved in discussion with a third author (AS). From the preclinical studies, the following information was extracted: (a) first author, (b) publication year, (c) study design, (d) treatment model, (e) treatment site, (f) species, (g) HyA application form, (h) treatment groups, (i) follow-up period, (j) available outcome parameters, and (k) funding details. Similarly, the following information was extracted from the clinical trials: (a) first author, (b) publication year, (c) study design, (d) patient characteristics (i.e., gender, age, health and smoking status), (e) site-specific inclusion criteria, (f) number of sites at baseline and last follow-up, (g) treatment groups, (h) product details, (i) application form, (j) follow-up period, (k) postoperative medication, (l) available outcome parameters, (m) clinical setting (i.e., private practice or university setting), and (n) funding details. Finally, all available information on the HyA-based products was summarized, i.e., (a) trade name, (b) manufacturer, (c) concentration, (d) chemical form, and (e) application form.
Risk of bias assessment
For the preclinical trials, the SYRCLE’s risk of bias (RoB) tool was used [20]. As suggested, the following criteria were evaluated as having “low,” “high,” or “unclear” RoB: (1) sequence generation, (2) baseline characteristics, (3) allocation concealment, (4) random housing, (5) blinding caregivers or researchers, (6) random outcome assessment, (7) blinding outcome assessor, (8) incomplete outcome data, (9) selective outcome reporting, and (10) other sources of bias. For each study, the number and percentage of positively scored items were calculated (i.e., “quality score”).
For the RCT, the Cochrane Collaboration’s RoB 2.0 tool was used [21]. The RoB was judged as having “low,” “high,” or “some” concerns for each of the following criteria: (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, (5) selection of the reported result, and (6) overall risk of bias. For the non-randomized trials, the ROBINS-I tool was used [22]. The risk of bias was judged as “low,” “moderate,” “serious,” “critical,” or “no information” for the following criteria: (1) confounding, (2) selection of participants, (3) classification of interventions, (4) deviations from intended interventions, (5) missing outcome data, (6) measurement of the outcome, (7) selection of the reported result, and (8) overall risk of bias.
The assessment was done by 2 reviewers (DD, KB), and in case of any ambiguity consensus was achieved by discussion with a third author (AS). One author repeated the assessment (DD).
Synthesis of results and statistical analysis
For the preclinical studies, new bone formation and bone volume per tissue volume (BV/TV) were considered main outcome parameters, while for the clinical studies pain, trismus, and swelling were defined as main outcome parameters. Data were extracted from the text, tables, and figures, calculated, and/or the authors of the original publications were contacted.
In case at least 2 randomized studies with comparable study design (i.e., treatment indication, HyA regime, follow-up period, outcome assessment) were identified, a pairwise meta-analysis was performed. The meta-analyses were limited to RCTs, thus including studies of greater methodological quality. The groups applying HyA were either compared to a negative control group (i.e., with no additional treatment step) or to a control group applying another treatment, including a placebo or the carrier material of the test group (“placebo/carrier”). Pairwise meta-analyses were performed for each separate comparison as well as overall. Restricted maximum likelihood to calculate heterogeneity (τ2) was used and the Knapp–Hartung standard error adjustment to account for the small number of studies. The mean difference between control and test group, the standard error of the mean difference, and 95% confidence interval (CI) were calculated. In studies using split-mouth design, the data were treated as dependent when calculating the standard error of the mean difference with setting r = 0.5. The chi-square test was used to assess heterogeneity, and a p-value < 0.1 was considered indicative of significant heterogeneity [23]. Further, I2 test for homogeneity was undertaken to quantify the extent of heterogeneity and in case of at least 3 comparable studies the 95% prediction interval was additionally calculated. Statistical analysis was performed with STATA/IC 17.0 for Mac.
Quality of evidence (GRADE)
The certainty of meta-analytic evidence of preclinical and clinical trials included herein was summarized by Grading of Recommendations Assessment, Development and Evaluation (GRADE) [24, 25]. For both preclinical and clinical trials, the GRADEpro GDT (Guideline Development Tool, McMaster University and Evidence Prime, 2022) software was used to grade the quality of evidence of the results.
Results
Study selection and characteristics
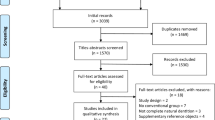
The literature search is presented in the Appendix 3; 147 potential references were identified and, after removing the duplicates, 90 studies were left for title and abstract screening. A total of 57 studies were removed for various reasons leaving 33 studies for full text analysis. After excluding another 6 studies in which the product type did not meet the inclusion criteria or incorrect study design [26,27,28,29], 5 preclinical and 22 clinical studies were included in the present systematic review. Both reviewers agreed perfectly on studies chosen for full-text screening (Cohen’s kappa = 1; 100% agreement), while substantial agreement was achieved for final study enrollment (Cohen’s kappa = 0.61; 84.9% agreement).
In all preclinical trials, HyA was applied into the tooth socket after extraction of regularly erupted teeth [30,31,32,33,34]. The clinical trials were divided into 3 groups according to treatment indication: (1) surgical removal of LM3 (RCT (n = 10), CT (n = 1)) [35,36,37,38,39,40,41,42,43,44,45], (2) extraction of regularly erupted teeth (RCT (n = 7), non-randomized split-mouth study (n = 1), prospective case series (n = 1)) [46,47,48,49,50,51,52,53,54], and (3) treatment of AO (RCT (n = 1), prospective case series (n = 1)) [55, 56].
Study population
Regarding the preclinical studies, 2 studies included 5–11 Holtzman or 5–6 Wistar rats in the various groups, respectively [30, 31], while 3 studies used beagle dogs (20 dogs in total) [32,33,34]. In the rat studies, HyA was applied in the extraction socket in either healthy or diabetic animals, whereas in the dog studies HyA was applied in infected extraction sockets (Table 1).
The clinical studies on surgical LM3 removal, extraction of regularly erupted teeth, and treatment of AO included at final evaluation 603, 349, and 110 patients, respectively, contributing with 306, 226, and 90 HyA treated sites, and 370, 257, and 20 control/non-HyA treated sites, respectively (Table 2). In most of the studies, patients were systemically healthy, while one study each regarded patients with either chronic liver disease or diabetics; 4 studies did not report on patients’ health status. Smoking status was reported in 12 studies; 8 studies included only non-smokers, 2 studies included patients smoking ≤ 10 cigarettes/day, and 2 studies included both, i.e., non-smokers and smokers. Ten studies did not provide any information on smoking status.
In the studies on LM3 extraction, the teeth were asymptomatic, predominantly vertically impacted or half impacted allowing primary wound closure after surgical removal. Half of the studies on extraction of regularly erupted teeth included only single rooted teeth (either anterior teeth or premolars), whereas the other half included either molars or any type of tooth. Both studies in the AO treatment group included all tooth types fulfilling the criteria of AO according to Blum et al. (2002) [57].
Study intervention
In all preclinical trials, HyA was applied as a gel into the extraction socket directly after tooth removal either alone (n = 3) or in combination with an absorbable collagen sponge (n = 2) (Table 1).
In most clinical studies (n = 19) (Table 2), HyA was applied as a gel intra-operatively into the extraction socket or post-operatively at the extraction site either alone (n = 13) or with some carrier ((i.e., absorbable collagen sponge (n = 3), leukocyte- and platelet-rich fibrin (n = 2), or bone substitutes (n = 1)). In the remaining 3 clinical studies, HyA was either used as spray 3 times per day for 1 week (n = 2) or as mouthwash (n = 1).
HyA information
In the 5 preclinical and 22 clinical studies included, 11 commercial, 2 self-made, and 2 of unknown origin HyA products were used (Table 3). In all preclinical studies (n = 5), HyA was applied as a gel, while in the clinical studies HyA was applied as gel (n = 15), spray (n = 2), mouthwash (n = 1), or combined with a sponge (n = 3) or bone substitute material (n = 1) during the fabrication process. The concentration of HyA varied from 0.2% in a spray, 0.25% in a mouthwash, up to 2.5% in a self-combined HyA sponge, while in 5 studies the concentration of HyA was not reported. The chemical form, i.e., non-cross-linked or cross-linked, was not reported in most of the studies (n = 16), whereas 10 studies used non-cross-linked HyA, and one study combined non- and cross-linked HyA.
Clinical setting and funding details
All preclinical trials were funded by independent single [32, 33, 58] or multiple research grants [30, 59].
In one clinical study, a multicenter study design was reported including 8 medical centers [56], whereas all other clinical studies were performed in a single department in a university setting. Eleven clinical studies did not report on funding sources, while in 9 clinical studies [35, 36, 42, 44, 45, 52, 53, 56, 60] the funding was provided by the department; however, in 3 out of these 9 studies, the HyA gel was provided by the manufacturer [46, 52, 53]. In a single study, the funding was provided by 3 different research foundations [48].
Reported outcome variables and follow-up
In the preclinical studies, bone formation was assessed by different methods between 14 days and 3 months postoperatively. One study investigated in addition the level of bone morphogenetic protein-2 and osteopontin (Table 1). Furthermore, 4 studies recorded no side effect after HyA application, while one study did not report absences/presence of side effects.
In the clinical studies, the evaluated outcome parameters varied depending on treatment indication (Table 2). In the studies on surgical removal of LM3, presence of pain measured by visual analogue scale (VAS), swelling, and trismus were the outcome parameters most often evaluated. Other less frequently assessed parameters were presence/absence of prolonged bleeding, presence/absence of soft tissue dehiscence, speed of mucosal healing, rate of AO/wound infection, and laboratory markers of inflammation, oxidative stress, and wound healing. Among the studies on extraction of regularly erupted teeth, 3 publications used different socket-/soft tissue healing scores, 3 publications assessed the amount of newly formed bone and/or alveolar dimensional changes, 3 publications assessed pain, and one study assessed the rate of AO. Both studies on AO treatment focused on assessment of pain and adverse reactions. Most of the clinical studies recorded no side effects after local application of HyA, while 6 studies did not mention the absences/presence of side effects. A single study [37] applying 0.8% HyA gel after LM3 removal reported a significantly prolonged bleeding time after wound closure compared to the control group; however, as hemostasis was within a physiological timeframe, this was not considered an adverse event.
Summary of the results of the individual studies
In all preclinical studies (Table 4), based on histologic, radiologic, or immunohistochemical analysis, the test groups with HyA showed significantly better results compared to the control group in at least one of the parameters regarding bone formation; this was independent of socket condition (healthy or infected) and type of control treatment.
In 4 out of 10 clinical studies on surgical LM3 removal (Table 5) reporting on pain, significant advantages for the test group using HyA, compared to the control group, were reported in at least one postoperative timepoint. Similarly, in 4 out of 7 studies and in 3 out of 9 studies reporting on swelling and trismus, respectively, significant advantages in favor of HyA application compared to the control group were reported. In 3 out of 4 studies reporting on soft tissue healing after extraction of regularly erupted teeth, significantly improved soft tissue healing after HyA application compared to the control group was recorded. Furthermore, one study reported improved bone formation after 30 days, one study reported a decreased reduction of alveolar diameter after up to 21 days, while 2 studies reported either no difference between the groups or significant disadvantages for the test group using HyA in terms of alveolar dimensional changes. Finally, pain perception was reported in 6 studies, but only 2 studies reported significant differences between the groups in favor of HyA application. One study assessing treatment of AO reported significantly lower postoperative pain after HyA application compared to the application of alvogyl; the second study had no control group.
Synthesis of results
Preclinical studies—bone volume per tissue volume in preclinical trials
Two preclinical studies provided data to summarize radiographically assessed BV/TV 3 months postoperatively [33, 58] (Fig. 1). The studies compared the application of HyA in combination with an absorbable collagen sponge versus the absorbable collagen sponge. Overall, no significant difference between the groups was identified (effect size: 9.57; 95% CI: − 86.22 to 105.36; p = 0.42), but statistical heterogeneity among the studies was significant (I2 = 89.89%; p < 0.01).
Clinical studies—evaluation of pain 2–3 and 7 days after surgical LM3 removal
Based on the results of 4 RCTs [37, 39, 40, 61], perception of pain showed no statistical significant differences between test and control groups 2–3 days postoperatively (effect size: 0.52; 95% CI: − 0.34–1.38; p = 0.15), without statistical heterogeneity among the studies (I2 = 0.00%; p = 0.37). Separate analyses with 2 studies each comparing HyA with a negative control group (effect size: 0.44; 95% CI: − 3.24–4.12; p = 0.37) and HyA with a placebo/carrier group (effect size: 0.78; 95% CI: − 7.67–9.24; p = 0.45) lacked also statistical significance (Fig. 2a).
Based on the results of 5 RCTs [36, 37, 39, 40, 61], perception of pain 7 days postoperatively was significantly lower in the test groups applying HyA (effect size: 0.32; 95% CI: 0.12–0.51; p = 0.01), without statistical heterogeneity among the studies (I2 = 0.00%; p = 0.84). However, the separate analyses lacked statistical significance for the comparison HyA with a negative control group (3 studies; effect size: 0.27; 95% CI: − 0.05–0.60; p = 0.07) and for the comparison HyA with a placebo/carrier group (2 studies; effect size: 0.53; 95% CI: − 0.48–1.54; p = 0.09; Fig. 2b).
Clinical studies—evaluation of swelling 2–3 and 7 days after surgical LM3 removal
Based on the results of 2 RCT [37, 39], the extent of swelling 2–3 days postoperatively showed no significant difference between test and control groups (effect size: − 2.08; 95% CI: − 23.73–19.58; p = 0.44); however, statistical heterogeneity among the studies was significant (I2 = 87.94%; p < 0.01; Fig. 3a).
Forest plot on the effect size of HyA application (test) on swelling after surgical LM3 removal 2–3 days (a) and 7 days (b) postoperatively. The values of both studies are based on a length measurement (i.e., from the ear to the corner of the mouth in mm); please note that the original data set has been provided by Bayoumi et al.
Similarly, the extent of swelling 7 days postoperatively also showed no significant difference between test and control groups (effect size: 1.75; 95% CI: − 14.38–17.89; p = 0.40), and statistical heterogeneity among the studies was again significant (I2 = 66.86%; p = 0.08; Fig. 3b). No separate analysis for the comparison HyA with either a negative control or placebo/carrier group was possible due to the limited number of studies.
Clinical studies—evaluation of trismus 2–3 and 7 days after surgical LM3 removal
Based on the results of 3 RCTs [37, 39, 41], trismus showed 2–3 days postoperatively no significant differences between test and control groups (effect size: 1.31; 95% CI: − 0.65–3.26; p = 0.10), without statistical heterogeneity among the studies (I2 = 25.86%; p = 0.37). The separate analyses also lacked statistical significance for the comparison HyA with a negative control group (2 studies; effect size: 1.33; 95% CI: − 7.25–9.91; p = 0.30), while only a single study was available for the comparison HyA with a placebo/carrier group (Fig. 4a).
Based on the results of 5 RCTs [36, 37, 39,40,41], trismus showed no significant difference between test and control groups 7 days postoperatively (effect size: 1.08; 95% CI: − 0.97–3.12; p = 0.22); however, statistical heterogeneity among the studies was significant (I2 = 55.99%; p = 0.07). The separate analyses also showed no significant difference between HyA and a negative control group (4 studies; effect size: 0.51; 95% CI: − 0.29–1.32; p = 0.14), while only a single study was available for the comparison HyA with a placebo/carrier group (Fig. 4b).
Risk of bias assessment
Among the preclinical studies, the quality score ranged between 20 and 40% (Appendix 4); only reporting of baseline characteristics and other sources of bias were judged in all studies as low risk of bias.
The included RCT were either judged as having some concerns (n = 13) or low risk of bias (n = 5) (Appendix 5). None of the RCT deviated from the intended intervention, 5 RCTs were judged as having some concerns in the randomization process, and approximately half of studies were judged as having some concerns in their reporting on missing outcome data, measurement of the outcome, and selection of the reported results. Most of the non-randomized studies were judged as having a low risk of bias (n = 3), whereas one study was judged as having some concerns (Appendix 6).
Quality of evidence (GRADE)
For the results of the meta-analysis including 2 preclinical trials, the certainty of evidence for the outcome parameter BV/TV after 3 months was rated low (Appendix 8a).
The certainty of evidence obtained from meta-analyses including clinical trials was judged as moderate for pain perception and trismus and as low for the swelling assessment (Appendix 8b).
Discussion
HyA has been shown to possess anti-inflammatory, anti-edematous, osteoinductive, and pro-angiogenetic properties; thus, it appears that HyA improves wound healing [62,63,64,65]. The present systematic review aimed to provide a comprehensive assessment of all available evidence (i.e., preclinical and clinical) on the effect of HyA application in connection with tooth extraction. Overall, it seems that HyA application in connection to surgical LM3 removal may have a positive effect in pain reduction during the first post-operative week. Specifically, meta-analysis of 5 clinical studies showed that local (intra-surgical) application of HyA gel was associated with a statistically significantly reduced perception of pain 7 days postoperatively compared to the control group with either no additional wound manipulation or the application of a placebo/carrier. HyA application did not seem to have any impact on other often appearing complications after LM3 removal (i.e., swelling and trismus) or in connection with non-surgical extraction of normally erupted teeth.
This positive effect of intra-surgical application of HyA on pain perception within the first post-operative week of LM3 removal adds on the results of a previous systematic review, which also assessed the possible benefit of HyA in the same indication [17]. Specifically, based on a different study selection, HyA application significantly reduced pain on both the 3rd and 7th postoperative day [17]. Apparently, the positive effect of HyA in the very early post-operative days observed in that review was not seen in the present meta-analysis, due to the increased information provided by 2 additional studies [39, 61] included herein and due to the exclusion of a non-randomized study, which strongly favored the HyA test group [44]. A positive effect of HyA in terms of reduced pain perception can be partly explained by its modulating effect on the inflammatory response at the surgical site. It has been previously demonstrated that HyA can downregulate the production and expression of prostaglandin E2, bradykinin, and substance P, which are all involved in pain transmission and sensation [66]. Nevertheless, any potential positive effect of HyA on the local inflammatory response does not necessarily translate in less swelling and/or trismus in the clinic, since both the analyses included herein and those in the above-mentioned review failed to indicate any differences between test and control groups regarding these aspects. However, these results should be interpreted with care due to the small number of original studies and the lack of standardization in the methods assessing facial swelling as well as in the intervention per se. For example, the included studies seldomly provided information on the level of surgical difficulty and/or applied flap design, aspects which may affect the outcome parameters [67]. Moreover, the lack of any significant positive effect of HyA in pain perception in non-surgical extraction of regularly erupted teeth, seen in most studies (4 out 5) included in this review, should not be interpreted as lack of action of HyA per se. It may be due differences in the healing mode, i.e., “closed” after surgical LM3 removal versus “open” after extraction of regularly erupted teeth, where the lack of primary closure and of any carrier may have resulted in a fast wash-out of HyA. Whether the application of HyA in a carrier could improve its action, is difficult to assess, as this was used only in a single study that failed to show any differences [47]. Nevertheless, it should also be kept in mind that in most cases, uncomplicated tooth extraction is associated with low levels of pain, and thus any possible positive effect of HyA may be difficult to capture. In fact, in the only comparative study on AO management included in this review, significantly reduced pain postoperatively in the groups receiving HyA (with no primary closure and no use of a carrier) was reported.
Some of the studies on healing after extraction of regularly erupted teeth, included in this review, also assessed the possible impact of HyA application on soft and hard tissue healing. In 3 out of 4 studies assessing soft tissue healing, a positive effect of HyA was reported based on the time until and/or percentage of socket closure, as well as based on scores for judging soft tissue healing. In contrast, in 3 comparative studies, intra- or post-operative use of HyA gel did not have any positive effect in terms of alveolar dimensional changes compared to no HyA application, after a follow-up time of 3 to 4 months [48, 52, 53]. In fact, in one of the studies [52], where following ridge preservation with socket grafting with collagen-enriched, deproteinized bovine bone mineral and socket sealing by means of a collagen matrix surgical therapy, HyA gel was applied onto the collagen matrix three times per day for 1 week, significantly more horizontal bone loss at the coronal aspect of the extraction sockets was observed. These findings on lack of a positive effect of HyA on bone may appear somehow in contrast with the findings reported in the preclinical studies included herein. In the 2 studies reporting on healing of non-infected extraction sockets in either healthy [30] or diabetic [31] rats, HyA application significantly enhanced bone healing compared to the control group. Similarly, in 3 out of 3 dog studies reporting on healing of infected extraction sockets, HyA application either alone [32] or with a collagen sponge [33, 58] or deproteinized bovine bone mineral with collagen as carrier [58] enhanced bone healing. It is important to mention, however, that this positive effect of HyA on bone healing was not shown in the only meta-analysis possible herein regarding BV/TV, probably due to the fact that both studies used a late healing time for this particular animal model; i.e., bone healing inside an extraction socket in the dog is rather advanced after 3 months, even without any treatment [68]. Noteworthy, BV/TV in the HyA group was similar to that in another test group, treated with recombinant human bone morphogenetic protein-2 (rhBMP-2), a known very potent bone enhancing agent [33, 58]. Furthermore, such positive effects of HyA on bone healing have also been shown in other preclinical studies, using critical size defect models [13, 14]. In perspective, no study on surgical removal of LM3 assessed the healing outcome at the distal aspect of the lower second molar, a site that is often associated with a deep periodontal defect after extraction of impacted LM3 [5].
This review tried also to identify whether application of HyA may reduce the rate of AO after tooth extraction; however, there was limited reporting on this complication in the studies. In this context, application of HyA is in general considered safe and with no side effects; however, it must be mentioned that HyA may lead to significant adverse events in case it is applied (injected) within the tissues [69]. Herein, only a single study [37] reported a prolonged bleeding time after wound closure compared to the control group; however, hemostasis was judged to be within a physiological timeframe and therefore not considered an adverse event. All other studies included in this review did not mention any side effects or complications after HyA application. Besides the fact that HyA is safe to apply in connection with surgical LM3 or non-surgical tooth extraction, no conclusions can be made regarding the most efficient HyA formulation (e.g., low vs. high concentration, non-cross-linked vs. cross-linked, gel vs. spray) or application mode (e.g., with vs. without a carrier, frequency), and thus no clear recommendation can be provided.
Altogether, only a limited number of well-designed, randomized preclinical and clinical trials could be identified herein and combined in a meta-analysis. Moreover, as outlined above, there is a lack of consensus and information on HyA product details, but also on the surgical details (e.g., level of surgical difficulty or flap design). These limitations resulted in an overall low to moderate certainty of evidence. In future studies, a better and more standardized reporting on HyA product details, dosage, and application, and longer follow-up times should be implemented to allow for a more complete evaluation of the potential of HyA use in connection with tooth extraction. In addition, future updated systematic reviews including a larger number of studies should also consider in the meta-analyses a comparison between studies with parallel arms and studies in split-mouth design. This would be specifically of interest for parameters such as pain perception, something not feasible herein due to the very limited number of split-mouth studies [39, 41].
Conclusion
The results of the present systematic review and meta-analyses showed that intra-surgical application of HyA in connection with surgical LM3 removal resulted in significant reduction in pain perception 7 days postoperatively, while early post-operative pain, trismus, and extent of swelling were unaffected. Furthermore, it seems that HyA application may have a positive effect on soft tissue healing after non-surgical extraction of normally erupted teeth, but it seems not to reduce post-extraction alveolar ridge modeling even though evidence from preclinical studies indicated that HyA may enhance bone formation.
Data availability
Data are available from the authors upon reasonable request.
References
Upadhyaya C, Humagain H (2010) Prevalence of dry socket following extraction of permanent teeth at Kathmandu University Teaching Hospital (KUTH), Dhulikhel, Kavre, Nepal: a study. Kathmandu Univ Med J (KUMJ) 8(29):18–24
Akinbami BO, Godspower T (2014) Dry socket: incidence, clinical features, and predisposing factors. Int J Dent 2014:796102
Duarte-Rodrigues L et al (2018) Third molar removal and its impact on quality of life: systematic review and meta-analysis. Qual Life Res 27(10):2477–2489
Halabi D et al (2018) Chlorhexidine for prevention of alveolar osteitis: a randomised clinical trial. J Appl Oral Sci 26:e20170245
Kan KW et al (2002) Residual periodontal defects distal to the mandibular second molar 6–36 months after impacted third molar extraction. J Clin Periodontol 29(11):1004–1011
Peng KY et al (2001) Mandibular second molar periodontal status after third molar extraction. J Periodontol 72(12):1647–1651
MacBeth N et al (2017) Hard and soft tissue changes following alveolar ridge preservation: a systematic review. Clin Oral Implants Res 28(8):982–1004
Taberner-Vallverdú M et al (2015) Efficacy of different methods used for dry socket management: a systematic review. Med Oral Patol Oral Cir Bucal 20(5):e633–e639
Taberner-Vallverdú M, Sánchez-Garcés M, Gay-Escoda C (2017) Efficacy of different methods used for dry socket prevention and risk factor analysis: a systematic review. Med Oral Patol Oral Cir Bucal 22(6):e750–e758
Han W et al (2022) The anti-inflammatory activity of specific-sized hyaluronic acid oligosaccharides. Carbohydr Polym 276:118699
Harris LG, Richards RG (2004) Staphylococcus aureus adhesion to different treated titanium surfaces. J Mater Sci Mater Med 15(4):311–314
Romanò CL et al (2017) Hyaluronic acid and its composites as a local antimicrobial/antiadhesive barrier. J Bone Jt Infect 2(1):63–72
Matheus HR et al (2021) Association of hyaluronic acid with a deproteinized bovine graft improves bone repair and increases bone formation in critical-size bone defects. J Periodontol 92(11):1646–1658
Yun J, Lee J, Ha CW et al (2021) The effect of 3-D printed polylactic acid scaffold with and without hyaluronic acid on bone regeneration. J Periodontol 1–11. https://doi.org/10.1002/JPER.21-0428
Shirakata Y et al (2021) Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with cross-linked hyaluronic acid-gel with or without a collagen matrix: a preclinical study in dogs. Quintessence Int 0(0):308–316
Shirakata Y et al (2021) Healing of buccal gingival recessions following treatment with coronally advanced flap alone or combined with a cross-linked hyaluronic acid gel. An experimental study in dogs. J Clin Periodontol 48(4):570–580
Maria de Souza G et al (2020) The effectiveness of hyaluronic acid in controlling pain, edema, and trismus after extraction of third molars: systematic review and meta-analysis. J Oral Maxillofac Surg 14:14
de Vries R et al (2015) A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid-Based Preclin Med 2:e00007
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10(1):89
Hooijmans CR et al (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:43
Sterne JAC et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Sterne JA et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Higgins JPT, Thompson SG, Spiegelhalter DJ (2009) A re-evaluation of random-effects meta-analysis. J R Stat Soc A Stat Soc 172(1):137–159
Wei D et al (2016) The use of GRADE approach in systematic reviews of animal studies. J Evid Based Med 9(2):98–104
Chen H et al (2015) How to use gradepro gdt to rate the quality of evidence in systematic reviews of intervention studies: An introduction. Chin J Evid Based Med 15:600–606
Mendes RM et al (2010) Effects of single wall carbon nanotubes and its functionalization with sodium hyaluronate on bone repair. Life Sci 87(7–8):215–222
Kapitan M et al (2021) Initial observation of factors interfering with the treatment of alveolar osteitis using hyaluronic acid with octenidine-a series of case reports. Biomolecules 11(8):04
Martins-Junior PA et al (2016) Evaluation of carbon nanotubes functionalized with sodium hyaluronate in the inflammatory processes for oral regenerative medicine applications. Clin Oral Invest 20(7):1607–1616
Catanzano O et al (2018) Composite alginate-hyaluronan sponges for the delivery of tranexamic acid in postextractive alveolar wounds. J Pharm Sci 107(2):654–661
Mendes RM et al (2008) Sodium hyaluronate accelerates the healing process in tooth sockets of rats. Arch Oral Biol 53(12):1155–1162
Sa MA et al (2013) Carbon nanotubes functionalized with sodium hyaluronate restore bone repair in diabetic rat sockets. Oral Dis 19(5):484–493
Kim JJ et al (2016) Hyaluronic acid improves bone formation in extraction sockets with chronic pathology: a pilot study in dogs. J Periodontol 87(7):790–795
Kim JJ et al (2019) Biomodification of compromised extraction sockets using hyaluronic acid and rhBMP-2: an experimental study in dogs. J Periodontol 90(4):416–424
Lee JB et al (2021) Effects of hyaluronic acid and deproteinized bovine bone mineral with 10% collagen for ridge preservation in compromised extraction sockets. J Periodontol 23:23
Koray M et al (2014) Efficacy of hyaluronic acid spray on swelling, pain, and trismus after surgical extraction of impacted mandibular third molars. Int J Oral Maxillofac Surg 43(11):1399–1403
Gocmen G et al (2015) The antioxidant and anti-inflammatory efficiency of hyaluronic acid after third molar extraction. J Craniomaxillofac Surg 43(7):1033–1037
Gocmen G et al (2017) Effects of hyaluronic acid on bleeding following third molar extraction. J Appl Oral Sci 25(2):211–216
Afat İM, Akdoğan ET, Gönül O (2018) Effects of leukocyte- and platelet-rich fibrin alone and combined with hyaluronic acid on pain, edema, and trismus after surgical extraction of impacted mandibular third molars. J Oral Maxillofac Surg 76(5):926–932
Bayoum A, Nadershah M, Albandar A, Alsulaimani B, Sankour I et al (2018) The effect of cross-linked hyaluronic acid in surgical extraction of impacted mandibular third molars. Int J Dent Oral Health 4(2). https://doi.org/10.16966/2378-7090.254
Guazzo R et al (2018) Effect on wound healing of a topical gel containing amino acid and sodium hyaluronate applied to the alveolar socket after mandibular third molar extraction: A double-blind randomized controlled trial. Quintessence Int 49(10):831–840
Merchant R et al (2018) Comparative evaluation of clinical efficacy of hyaluronic acid spray versus normal saline spray on swelling, pain, and trimus after surgical extraction of impacted mandibular third molar – a randomized controlled split mouth study. Int J Sci Res 7:152
Afat IM, Akdoğan ET, Gönül O (2019) Effects of leukocyte- and platelet-rich fibrin alone and combined with hyaluronic acid on early soft tissue healing after surgical extraction of impacted mandibular third molars: a prospective clinical study. J Craniomaxillofac Surg 47(2):280–286
Munoz-Camara D, Pardo-Zamora G, Camacho-Alonso F (2020) Postoperative effects of intra-alveolar application of 0.2% chlorhexidine or 1% hyaluronic acid bioadhesive gels after mandibular third molar extraction: a double-blind randomized controlled clinical trial. Clin Oral Investig 24:24
Yilmaz N et al (2017) The efficacy of hyaluronic acid in postextraction sockets of impacted third molars: a pilot study. Niger J Clin Pract 20(12):1626–1631
Yang H et al (2020) Non-inferiority study of the efficacy of two hyaluronic acid products in post-extraction sockets of impacted third molars. Maxillofac Plast Reconstr Surg 42(1):40
Favia G et al (2008) Accelerated wound healing of oral soft tissues and angiogenic effect induced by a pool of aminoacids combined to sodium hyaluronate (AMINOGAM®). J Biol Regul Homeost Agents 22(2):109–116
Bayoumi AM, Jan A, Amoudi WA, Shakir M (2015) The Effects of Using Hyaluronic Acid on the Extraction Sockets. Int J Dent Oral Health 2(1). https://doi.org/10.16966/2378-7090.157
Alcântara CEP et al (2018) Hyaluronic acid accelerates bone repair in human dental sockets: a randomized triple-blind clinical trial. Braz Oral Res 32:e84
Lorenz J et al (2018) Injectable bone substitute material on the basis of beta-TCP and hyaluronan achieves complete bone regeneration while undergoing nearly complete degradation. Int J Oral Maxillofac Implants 33(3):636–644
Marin S et al (2020) Hyaluronic acid treatment outcome on the post-extraction wound healing in patients with poorly controlled type 2 diabetes: a randomized controlled split-mouth study. Med Oral Patol Oral Cir Bucal 25(2):e154–e160
Mostafa D et al (2021) Effect of hyaluronic acid gel on healing of simple dental extraction sockets: a pilot study. Open Access Maced J Med Sci 9(D):190–195
Eeckhout C et al (2022) A randomized controlled trial evaluating hyaluronic acid gel as wound healing agent in alveolar ridge preservation. J Clin Periodontol 49(3):280–291
Cosola S, Oldoini G, Boccuzzi M, Giammarinaro E, Genovesi A, Covani U, Marconcini S (2022) Amino acid-enriched formula for the post-operative care of extraction sockets evaluated by 3-D intraoral scanning. Int J Environ Res Public Health 19(6):3302. https://doi.org/10.3390/ijerph19063302
Cocero N et al (2019) Efficacy of sodium hyaluronate and synthetic aminoacids in postextractive socket in patients with liver failure: split mouth study. J Biol Regul Homeost Agents 33(6):1913–1919
Dubovina D et al (2016) The use of hyaluronic and aminocaproic acid in the treatment of alveolar osteitis. Vojnosanit Pregl 73(11):1010–1015
Suchánek J, Ivančaková RK, Mottl R, Browne KZ, Pilneyová KC, Pilbauerová N, Schmidt J, Suchánková Kleplová T (2019) Hyaluronic acid-based medical device for treatment of alveolar osteitis-clinical study. Int J Environ Res Public Health 16:3698. https://doi.org/10.3390/ijerph16193698
Blum IR (2002) Contemporary views on dry socket (alveolar osteitis): a clinical appraisal of standardization, aetiopathogenesis and management: a critical review. Int J Oral Maxillofac Surg 31(3):309–317
Lee J-B, Chu S, Amara HB et al (2021) The effects of hyaluronic acid and deproteinized bovine bone mineral with 10% collagen for ridge preservation in compromised extraction sockets. J Periodontol 1–12. https://doi.org/10.1002/JPER.20-0832
Sá MA et al (2013) Carbon nanotubes functionalized with sodium hyaluronate restore bone repair in diabetic rat sockets. Oral Dis 19(5):484–493
Marin S et al (2020) Hyaluronic acid treatment outcome on the post-extraction wound healing in patients with poorly controlled type 2 diabetes: a randomized controlled split-mouth study. Medicina Oral, Patologia Oral y Cirugia Bucal 25(2):e154–e160
Muñoz-Cámara D, Pardo-Zamora G, Camacho-Alonso F (2021) Postoperative effects of intra-alveolar application of 0.2% chlorhexidine or 1% hyaluronic acid bioadhesive gels after mandibular third molar extraction: a double-blind randomized controlled clinical trial. Clin Oral Investig 25(2):617–625
de Brito Bezerra B et al (2012) Association of hyaluronic acid with a collagen scaffold may improve bone healing in critical-size bone defects. Clin Oral Implants Res 23(8):938–942
Raines AL et al (2011) Hyaluronic acid stimulates neovascularization during the regeneration of bone marrow after ablation. J Biomed Mater Res A 96(3):575–583
Turley EA, Noble PW, Bourguignon LY (2002) Signaling properties of hyaluronan receptors. J Biol Chem 277(7):4589–4592
Bertl K et al (2015) Hyaluronan in non-surgical and surgical periodontal therapy: a systematic review. J Clin Periodontol 42(3):236–246
Gupta RC et al (2019) Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front Vet Sci 6:192
Glera-Suárez P et al (2020) Patient morbidity after impacted third molar extraction with different flap designs. A systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal 25(2):e233–e239
Cardaropoli G, Araújo M, Lindhe J (2003) Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol 30(9):809–18
Bertl K et al (2017) Adverse reaction after hyaluronan injection for minimally invasive papilla volume augmentation a report on two cases. Clin Oral Implants Res 28(7):871–876
Acknowledgements
The authors wish to thank Prof. Amr Bayoumi (King Abdulaziz University, Saudi Arabia), who kindly provided additional information on his study.
Funding
Open access funding provided by Medical University of Vienna. Funding was solely provided by the authors’ institutions.
Author information
Authors and Affiliations
Contributions
Danijel Domic: study idea, data collection, data analysis, data interpretation, manuscript drafting.
Kristina Bertl: study idea, data collection, data analysis, data interpretation, manuscript drafting.
Tobias Lang: data collection, data analysis, manuscript revision.
Nikolaos Pandis: data analysis, data interpretation, manuscript revision.
Christian Ulm: study idea, data interpretation, manuscript revision.
Andreas Stavropoulos: study idea, data interpretation, manuscript drafting.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Domic, D., Bertl, K., Lang, T. et al. Hyaluronic acid in tooth extraction: a systematic review and meta-analysis of preclinical and clinical trials. Clin Oral Invest 27, 7209–7229 (2023). https://doi.org/10.1007/s00784-023-05227-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05227-4