Abstract
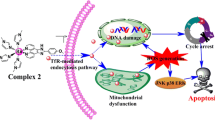
Induction of undesired toxicity and emergence of multidrug resistance (MDR) are the major obstacles for cancer treatment. Moreover, aggressive cancers are less sensitive towards existing chemotherapeutics. Therefore, selective targeting of cancers without inducing undesired side effects and designing proper strategies to overcome MDR has utmost importance in modern chemotherapy. Previously we revealed the anticancer properties of some transition metal chelates of Schiff base, but the effectiveness of nickel complex is still unrevealed. Herein, we synthesized and characterized a Schiff base nickel chelate, nickel-(II) N-(2-hydroxyacetophenone) glycinate (NiNG), through different spectroscopic means. NiNG proves to be a broad spectrum anticancer agent with considerable efficacy to overcome MDR in cancer. Antiproliferative effects of NiNG was evaluated using drug-resistant (CEM/ADR5000; NIH-MDR-G185; EAC/Dox), drug-sensitive aggressive (Hct116; CCRF-CEM; EAC/S) and normal (NIH-3T3) cells that reveal the selective nature of NiNG towards drug resistant and sensitive cancer cells without inducing any significant toxicity in normal cells. Moreover, NiNG involves reactive oxygen species (ROS)-mediated redox imbalance for induction of caspase 3-dependent apoptosis in aggressive drug-sensitive Hct116 and drug-resistant NIH-MDR-G185 cells through disruption of mitochondrial membrane potential. Moreover, intraperitoneal (i.p.) application of NiNG at non-toxic doses caused significant increase in the life-span of Swiss albino mice bearing sensitive and doxorubicin-resistant subline of Ehrlich ascites carcinoma cells. It is noteworthy that, in vitro NiNG can only overcome P-glycoprotein-mediated MDR while in vivo NiNG can overcome MRP1-mediated MDR in cancer. Therefore, NiNG has therapeutic potential to target and overcome MDR in cancer.
Graphical Abstract













Similar content being viewed by others
Abbreviations
- Ac-DEVD-cho:
-
N-Ac-Asp-Glu-Val-Asp-aldehyde
- ARD:
-
Acireductone dioxygenase
- ARDS:
-
Adult respiratory distress syndrome
- DMSO:
-
Dimethyl sulfoxide
- DTNB:
-
[5,5′-Dithio-bis-(2-nitrobenzoic acid)]
- MRP1:
-
Multidrug resistance protein 1
- NAC:
-
N-Acetyl cysteine
- Ni-SOD:
-
Nickel-superoxide dismutase
- PEG:
-
Polyethylene glycol
- z-VAD-fmk:
-
N-Benzyloxycarbonyl-Val-Ala-Asp(O-Me) fluoromethyl ketone
References
Ganguly A, Chakraborty P, Banerjee K, Choudhuri SK (2013) The role of a Schiff base scaffold, N-(2-hydroxy acetophenone) glycinate-in overcoming multidrug resistance in cancer. Eur J Pharm Sci 51:96–109
Haas KL, Franz KJ (2009) Application of metal coordination chemistry to explore and manipulate cell biology. Chem Rev 109:4921–4960
Fricker SP (2007) Metal based drugs: from serendipity to design. Dalton Trans 43:4903–4917
Meggers E (2009) Targeting proteins with metal complexes. Chem Commun (Camb) 9:1001–1010
Arjmand F, Aziz M (2009) Synthesis and characterization of dinuclear macrocyclic cobalt(II), copper(II) and zinc(II) complexes derived from 2,2,2′,2′-S,S[bis(bis-N,N-2-thiobenzimidazolyloxalato-1,2-ethane)]: DNA binding and cleavage studies. Eur J Med Chem 44:834–844
Pervez H, Manzoor N, Yaqub M, Khan A, Khan KM, Nasim FH, Choudhary MI (2010) Synthesis and urease inhibitory properties of some new N4-substituted 5-nitroisatin-3-thiosemicarbazones. Lett Drug Des Discov 7:102–108
Kovala-Demertzi D (2000) Transition metal complexes of diclofenac with potentially interesting anti-inflammatory activity. J Inorg Biochem 79:153–157
Thauer RK, Diekert G, Schönheit P (1980) Biological role of nickel. Trends Biochem Sci 5:304–306
Ragsdale SW (2009) Nickel-based enzyme systems. J Biol Chem 284:18571–18575
Anke M, Groppel B, Kronemann H, Grün M (1984) Nickel—an essential element. IARC Sci Publ 53:339–365
Welch RM (1981) The biological significance of nickel. J Plant Nutr 3:345–356
Denkhaus E, Salnikow K (2002) Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol Hematol 42:35–56
Sreekanth TVM, Nahajyothi PC, Lee KD, Prasad TNVKV (2013) Occurrence, physiological responses and toxicity of nickel in plants. Int J Environ Sci Technol 10:1129–1140
Cempel M, Nikel G (2006) Nickel: a review of its sources and environmental toxicology. Pol J Environ Stud 15:375–382
Das KK, Das SN, Dhundasi SA (2008) Nickel, its adverse health effects & oxidative stress. Indian J Med Res 128:412–425
Zhu T, Wang Y, Ding W, Xu J, Chen R, Xie J, Zhu W, Jia L, Ma T (2015) Anticancer activity and DNA-binding investigations of the Cu(II) and Ni(II) complexes with coumarin derivative. Chem Biol Drug Des 85:385–393
El-Tabl AS, El-Waheed MMA, Wahba MA, El-Fadl NAE-HA (2015) Synthesis, characterization, and anticancer activity of new metal complexes derived from 2-hydroxy-3-(hydroxyimino)-4-oxopentan-2-ylidene) benzohydrazide. Bioinorg Chem Appl 2015:1–14
Reed JE, Arnal AA, Neidle S, Vilar R (2006) Stabilization of G-quadruplex DNA and inhibition of telomerase activity by square-planar nickel(II) complexes. J Am Chem Soc 128:5992–5993
Drímal J, Zúrová-Nedelcevová J, Knezl V, Sotníková R, Navarová J (2006) Cardiovascular toxicity of the first line cancer chemotherapeutic agents: doxorubicin, cyclophosphamide, streptozotocin and bevacizumab. Neuro Endocrinol Lett 27:176–179
Sargent DJ, Niedzwiecki D, O’Connell MJ, Schilsky RL (2001) Recommendation for caution with irinotecan, fluorouracil, and leucovorin for colorectal cancer. N Engl J Med 345:144–145
Fine RL, Chabner BA (1986) In: Pinedo HM, Chabner BA (eds) Multidrug resistance. Elsevier, Amsterdam
Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM (1999) Biochemical, cellular and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39:361–398
Brost P, Elferink RO (2002) Mammalian ABC transporters in health and disease. Annu Rev Biochem 71:537–592
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
Kang YH, Yi MJ, Kim MJ, Park MT, Bae S, Kang CM, Cho CK, Park IC, Park MJ, Rhee CH, Hong SI, Chung HY, Lee YS, Lee SJ (2004) Caspase-independent cell death by arsenic trioxide in human cervical cancer cells: reactive oxygen species-mediated poly(ADP-ribose) polymerase-1 activation signals apoptosis-inducing factor release from mitochondria. Cancer Res 64:8960–8967
Half E, Arber N (2009) Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother 10:211–219
Boote DJ, Dennis IF, Twentyman PR, Osborne RJ, Laburte C, Hensel S (1996) Phase I study of etoposide with SDZ PSC 833 as a modulator of multidrug resistance in patients with cancer. J Clin Oncol 14:610–618
Kruijtzer CM, Beijnen JH, Rosing H, ten Bokkel Huinink WW, Schot M, Jewell RC (2002) Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol 20:2943–2950
Sandler A, Gordon M, De Alwis DP, Pouliquen I, Green L, Marder P (2004) A phase I trial of a potent P-glycoprotein inhibitor, zosuquidar trihydrochloride (LY335979), administered intravenously in combination with doxorubicin in patients with advanced malignancy. Clin Cancer Res 10:3265–3272
Stewart A, Steiner J, Mellows G, Laguda B, Norris D, Bevan P (2000) Phase I trial of XR9576 in healthy volunteers demonstrates modulation of P-glycoprotein in CD56+ lymphocytes after oral and intravenous administration. Clin Cancer Res 6:4186–4191
Tolcher AW, Cowan KH, Solomon D, Ognibene F, Goldspiel B, Chang R (1996) Phase I crossover study of paclitaxel with r-verapamil in patients with metastatic breast cancer. J Clin Oncol 14:1173–1184
Majumder S, Panda GS, Choudhuri SK (2003) Synthesis, characterization and biological properties of a novel copper complex. Eur J Med Chem 38:893–898
Ganguly A, Basu S, Chakraborty P, Chatterjee S, Sarkar A, Chatterjee M, Choudhuri SK (2010) Targeting mitochondrial cell death pathway to overcome drug resistance with a newly developed iron chelate. PLoS One 5:e11253
Ghosh RD, Das S, Ganguly A, Banerjee K, Chakraborty P, Sarkar A, Chatterjee M, Nanda A, Pradhan K, Choudhuri SK (2011) An in vitro and in vivo study of a novel zinc complex, zinc N-(2-hydroxyacetophenone)glycinate to overcome multidrug resistance in cancer. Dalton Trans 40:10873–10884
Ghosh RD, Banerjee K, Das S, Ganguly A, Chakraborty P, Sarkar A, Chatterjee M, Choudhuri SK (2013) A novel manganese complex, Mn-(II) N-(2-hydroxy acetophenone)glycinate overcomes multidrug-resistance in cancer. Eur J Pharm Sci 49:737–747
Sinha A, Banerjee K, Banerjee A, Das S, Choudhuri SK (2014) Synthesis, characterization and biological evaluation of a novel vanadium complex as a possible anticancer agent. J Organomet Chem 772–773:34–41
Ganguly A, Basu S, Banerjee K, Chakraborty P, Sarkar A, Chatterjee M, Choudhuri SK (2011) Redox active copper chelate overcomes multidrug resistance in T-lymphoblastic leukemia cell by triggering apoptosis. Mol BioSyst 7:1701–1712
Dakternieks D, Basu Baul TS, Dutta S, Tiekink ERT (1998) Synthesis, characterization, and X-ray structures of diphenyltin(IV) N-(2-hydroxyacetophenone) glycinate, its 1:1 adduct with triphenyltin(IV) chloride, and related systems. Organometallics 17:3058–3062
Kimmig A, Gekeler V, Neumann M, Frese G, Handgretinger R, Kardos G, Diddens H, Niethammer D (1990) Susceptibility of multidrug-resistant human leukemia cell lines to human interleukin 2-activated killer cells. Cancer Res 50:6793–6799
Efferth T, Konkimalla VB, Wang Y, Sauerbrey A, Meinhardt S, Zintl F, Mattern J, Volm M (2008) Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clin Cancer Res 14:2405–2412
Muscella A, Greco S, Elia MG, Storelli C, Marsigliante S (2002) Angiotensin II stimulation of Na/KATPase activity and cell growth by calcium-independent pathway in MCF-7 breast cancer cells. J Endocrinol 173:315–323
Currier SJ, Kane SE, Willingham MC, Cardarelli CO, Pastan I, Gottesman MM (1992) Identification of residues in the first cytoplasmic loop of P-glycoprotein involved in the function of chimeric human MDR1 MDR2 transporters. J Biol Chem 267:25153–25159
Banerjee K, Ganguly A, Chakraborty P, Sarkar A, Singh S, Chatterjee M, Bhattacharya S, Choudhuri SK (2014) ROS and RNS induced apoptosis through p53 and iNOS mediated pathway by a dibasic hydroxamic acid molecule in leukemia cells. Eur J Pharm Sci 52:146–164
Muscella A, Calabriso N, Fanizzi FP, De Pascali SA, Urso L, Ciccarese A, Migoni D, Marsigliante S (2008) [Pt(O, O0-acac)(c-acac)(DMS)], a new Pt compound exerting fast cytotoxicity in MCF-7 breast cancer cells via the mitochondrial apoptotic pathway. Br J Pharmacol 153:34–49
Deng WJ, Yang XQ, Liang YJ, Chen LM, Yan YY, Shuai XT, Fu LW (2007) FG020326-loaded nanoparticle with PEG and PDLLA improved pharmacodynamics of reversing multidrug resistance in vitro and in vivo. Acta Pharmacol Sin 6:913–920
Department of Health, Guidelines for the Testing for the Mutagenicity (1989) In: Report on Health and Social Subjects, London: HMSO, no. 35
Rao PV, Ashwini K, Ammani S (2007) Synthesis and characterization of transition metal complexes derived from some biologically active furoic acid hydrazones. Bull Chem Soc Ethiop 21:63–73
Clarke MJ (2003) Ruthenium metallopharmaceuticals. Coord Chem Rev 236:209–233
Iuch K, Hatano Y, Yagura T (2008) Heterocyclic organobismuth(III) induces apoptosis of human promyelocytic leukemic cells through activation of caspases and mitochondrial perturbation. Biochem Pharmacol 76:974–986
Giovanninia C, Matarreseb P, Scazzocchioa B, Sanchezc M, Masellaa R (2002) Mitochondria hyperpolarization is an early event in oxidized low-density lipoprotein-induced apoptosis in Caco-2 intestinal cells. FEBS Lett 523:200–206
Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, Los M (2009) Apoptosis and cancer: mutations within caspase genes. J Med Genet 46:497–510
Slee EA, Adrain C, Martin SJ (2001) Executioner Caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276:7320–7326
Fleury C, Mignotte B, Vayssiere JL (2002) Mitochondrial reactive oxygen species in cell death signalling. Biochimie 84:131–141
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8:579–591
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P (2006) Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 10:241–252
Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW, Peehl DM, Knox SJ (2002) Role of glutathione depletion and reactive oxygen species generation in apoptotic signalling in a human B lymphoma cell line. Cell Death Differ 9:252–263
Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V (2013) 8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications. Drug Des Devel Ther 7:1157–1178
Dhillon AS, Hagan S, Rath O, Kolch W (2007) MAP kinase signalling pathways in cancer. Oncogene 26:3279–3290
Timofeev O, Lee TY, Bulavin DV (2005) A subtle change in p38 MAPK activity is sufficient to suppress in vivo tumorigenesis. Cell Cycle 4:118–120
Torii S, Yamamoto T, Tsuchiya Y, Nishida E (2006) ERK MAP kinase in G cell cycle progression and cancer. Cancer Sci 97:697–702
Bragado P, Armesilla A, Silva A, Porras A (2007) Apoptosis by cisplatin requires p53 mediated p38α MAPK activation through ROS generation. Apoptosis 12:1733–1742
Newton R, Cambridge L, Hart LA, Stevens DA, Lindsay MA, Barnes PJ (2000) The MAP kinase inhibitors, PD098059, UO126 and SB203580, inhibits IL-1β-dependent PGE2 release via mechanistically distinct processes. Br J Pharmacol 130:1353–1361
Schaumloffel D (2012) Nickel species: analysis and toxic effects. J Trace Elem Med Biol 26:1–6
Landolph JR (1994) Molecular mechanisms of transformation of C3H/10T1/2 C18 mouse embryo cells and diploid human fibroblasts by carcinogenic metal compounds. Environ Health Perspect 102:119–125
Ziegler U, Groscurth P (2004) Morphological features of cell death. News Physiol Sci 19:124–128
Lavoie JN, Nguyen M, Marcellus RC, Branton PE, Shore GC (1998) E4orf4, a novel adenovirus death factor that induces p53-independent apoptosis by a pathway that is not inhibited by zVAD-fmk. J Cell Biol 140:637–645
Hadad J (2004) Redox and oxidant-mediated regulation of apoptosis signaling pathways: immuno-pharmaco-redox conception of oxidative siege versus cell death commitment. Int Immunopharmacol 4:475–493
Trompier D, Chang XB, Barattin R, du Moulinet D’Hardemare A, Di Pietro A, Baubichon-Cortay H (2004) Verapamil and its derivative trigger apoptosis through glutathione extrusion by multidrug resistance protein MRP1. Cancer Res 64:4950–4956
Das KK, Buchner V (2007) Effect of nickel exposure on peripheral tissues: role of oxidative stress in toxicity and possible protection by ascorbic acid. Rev Environ Health 22:157–173
Graham JA, Miller FJ, Daniels MJ, Payne EA, Gardner DE (1978) Influence of cadmium, nickel and chromium on primary immunity in mice. Environ Res 16:77–78
Sunderman FW Jr, Dingle B, Hopfer SM, Swift T (1988) Acute nickel toxicity in electroplating workers who accidentally ingested a solution of nickel sulphate and nickel chloride. Am J Ind Med 14:257–266
Ottolenghi AD, Haseman JK, Payne WW, Falk HL, MacFarlnad HN (1974) Inhalation studies of nickel sulphide in pulmonary carcinogenesis of rats. J Natl Cancer Inst 54:1165–1172
Acknowledgements
NIH-3T3 and NIH-MDR G185 cell lines were kindly provided by Dr. Michael M. Gottesman (National Cancer Institute, Bethesda, MD). CCRF-CEM and CEM/ADR5000 cell lines were kindly provided by Professor Thomas Efferth, University of Mainz, Germany. This investigation received financial support from the Indian Council of Medical Research (ICMR), New Delhi, No. 74/10/2014-PERS. (EMS). The funder had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Banerjee, K., Biswas, M.K. & Choudhuri, S.K. A newly synthesized nickel chelate can selectively target and overcome multidrug resistance in cancer through redox imbalance both in vivo and in vitro. J Biol Inorg Chem 22, 1223–1249 (2017). https://doi.org/10.1007/s00775-017-1498-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-017-1498-4