Abstract
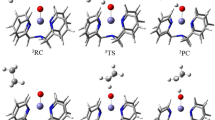
Through the introduction of dimethylamino (Me2N) substituent at the pyridine ring of 2-((R)-2-[(R)-1-(pyridine-2-ylmethyl)pyrrolidin-2-yl]pyrrolidin-1-ylmethyl)pyridine (PDP) ligand, the non-heme FeII(Me2NPDP)/H2O2/AcOH catalyst system was found to exhibit significant higher catalytic activity and enantioselectivity than the non-substituent one in the asymmetric epoxidation experiments. The mechanistic origin of the remarkable substituent effects in these oxidation reactions has not been well established. To ascertain the potent oxidant and the related reaction mechanism, a detailed DFT calculation was performed. Interestingly, a novel Fe(IV)-oxo Me2NPDP cation radical species, [(Me2NPDP)+ ·FeIV(O)(OAc)]2+ (Me2N 5), with about one spin spreading over the non-heme Me2NPDP ligand was formed via a carboxylic-acid-assisted O–O bond heterolysis, which is reminiscent of Compound I (an Fe(IV)(O)(porphyrin cation radical) species) in cytochrome P450 chemistry. Me2N 5 is energetically comparable with the cyclic ferric peracetate species Me2N 6, while in the pristine Fe(PDP) catalyst system, H 6 is more stable than H 5. Comparison of the activation energy for the ethylene epoxidation promoted by Me2N 5 and Me2N 6, Me2N 5 is supposed as the true oxidant triggering the epoxidation of olefins. In addition, a systematic research on the substituent effects varied from the electron-donating substituent (dMM, the substituents at sites 3, 4, and 5 of the pyridine ring: methyl, methoxyl, and methyl) to the electron-withdrawing one (CF3, 2,6-bis(trifluoromethyl)phenyl) on the electronic structure of the reaction intermediates has also been investigated. An alternative cyclic ferric peracetate complex is obtained, indicating that the substituents at the pyridine ring of PDP ligands have significant impacts on the electronic structure of the oxidants.











Similar content being viewed by others
References
Ortiz de Montellano PR (2005) Cytochrome P450: structure, mechanism, and biochemistry, 3rd edn. Kluwer Academic/Plenum Publishers, New York
Denisov IG, Makris TM, Sligar SG, Schlichting I (2005) Chem Rev 105(6):2253–2278
Zhang X, Li XX, Liu Y, Wang Y (2017) Front Chem 5:3
Li XX, Postils V, Sun W, Faponle AS, Sola M, Wang Y, Nam W, de Visser SP (2017) Chem Eur J 23(26):6406–6418
Wang Y, Kumar D, Yang C, Han K, Shaik S (2007) J Phys Chem B 111(26):7700–7710
Chakrabarty S, Austin RN, Deng D, Groves JT, Lipscomb JD (2007) J Am Chem Soc 129(12):3514–3515
Beauvais LG, Lippard SJ (2005) J Am Chem Soc 127(20):7370–7378
Merkx M, Kopp DA, Sazinsky MH, Blazyk JL, Müller J, Lippard SJ (2001) Angew Chem Int Ed 40(15):2782–2807
Que L Jr, Tolman WB (2008) Nature 455:333–340
Sun C-L, Li B-J, Shi Z-J (2011) Chem Rev 111(3):1293–1314
White MC (2012) Science 335:807–809
Hirao H, Kumar D, Que L Jr, Shaik S (2006) J Am Chem Soc 128(26):8590–8606
White MC, Doyle AG, Jacobsen EN (2001) J Am Chem Soc 123(29):7194–7195
Mas-Ballesté R, Que L Jr (2007) J Am Chem Soc 129(51):15964–15972
Chen MS, White MC (2007) Science 318:783–787
Chen MS, White MC (2010) Science 327:566–571
Cussó O, Cianfanelli M, Ribas X, Klein Gebbink RJ, Costas M (2016) J Am Chem Soc 138(8):2732–2738
Cussó O, Garcia-Bosch I, Ribas X, Lloret-Fillol J, Costas M (2013) J Am Chem Soc 135(39):14871–14878
Nishikawa Y, Yamamoto H (2011) J Am Chem Soc 133(22):8432–8435
Dubois G, Murphy A, Stack TDP (2003) Organ Lett 5(14):2469–2472
Marchi-Delapierre C, Jorge-Robin A, Thibon A, Menage S (2007) Chem Commun 11:1166–1168
Cussó O, Ribas X, Lloret-Fillol J, Costas M (2015) Angew Chem Int Ed 54(9):2729–2733
Miao C, Wang B, Wang Y, Xia C, Lee YM, Nam W, Sun W (2016) J Am Chem Soc 138(3):936–943
Lyakin OY, Bryliakov KP, Britovsek GJP, Talsi EP (2009) J Am Chem Soc 131(31):10798–10799
Lyakin OY, Bryliakov KP, Talsi EP (2011) Inorg Chem 50(12):5526–5538
Lyakin OY, Ottenbacher RV, Bryliakov KP, Talsi EP (2012) ACS Catal 2(6):1196–1202
Van Heuvelen KM, Fiedler AT, Shan X, De Hont RF, Meier KK, Bominaar EL, Münck E, Que L Jr (2012) Proc Natl Acad Sci USA 109(30):11933–11938
de Oliveira FT, Chanda A, Banerjee D, Shan X, Mondal S, Que L Jr, Bominaar EL, Münck E, Collins TJ (2007) Science 315:835–838
Wang Y, Janardanan D, Usharani D, Han K, Que L Jr, Shaik S (2013) ACS Catal 3(6):1334–1341
Oloo WN, Meier KK, Wang Y, Shaik S, Munck E, Que L Jr (2014) Nat Chem 5:3046
Makhlynets OV, Oloo WN, Moroz YS, Belaya IG, Palluccio TD, Filatov AS, Muller P, Cranswick MA, Que L Jr, Rybak-Akimova EV (2014) Chem Commun 50(6):645–648
Lyakin OY, Zima AM, Samsonenko DG, Bryliakov KP, Talsi EP (2015) ACS Catal 5(5):2702–2707
Zima AM, Lyakin OY, Ottenbacher RV, Bryliakov KP, Talsi EP (2016) ACS Catal 6(6):5399–5404
Zima AM, Lyakin OY, Ottenbacher RV, Bryliakov KP, Talsi EP (2017) ACS Catal 7(1):60–69
Osberger TJ, Rogness DC, Kohrt JT, Stepan AF, White MC (2016) Nature 537:214–219
Bigi MA, Liu P, Zou L, Houk KN, White MC (2012) Synlett 23(19):2768–2772
Vermeulen NA, Chen MS, Christina White M (2009) Tetrahedron 65(16):3078–3084
Bigi MA, Reed SA, White MC (2011) Nat Chem 3(3):216–222
Gormisky PE, White MC (2013) J Am Chem Soc 135(38):14052–14055
Shaik S, Kumar D, de Visser SP, Altun A, Thiel W (2005) Chem Rev 105(6):2279–2328
Shaik S, Cohen S, Wang Y, Chen H, Kumar D, Thiel W (2010) Chem Rev 110(2):949–1017
Cussó O, Garcia-Bosch I, Font D, Ribas X, Lloret-Fillol J, Costas M (2013) Organ Lett 15(24):6158–6161
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G, Nakatsuji H, Caricato M, Li X, Hratchian H, Izmaylov A, Bloino J, Zheng G, Sonnenberg J, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J, J. A., Peralta J, Ogliaro F, Bearpark M, Heyd J, Brothers E, Kudin K, Staroverov V, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J, Iyengar S, Tomasi J, Cossi M, Rega N, Millam J, Klene M, Knox J, Cross J, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R, Yazyev O, Austin A, Cammi R, Pomelli C, Ochterski J, Martin R, Morokuma K, Zakrzewski V, Voth G, Salvador P, Dannenberg J, Dapprich S, Daniels A, Farkas O, Foresman J, Ortiz J, Cioslowski J, Fox D (2010) Gaussian 09, Revision B.01. Gaussian, Inc., Wallingford
Becke AD (1992) J Chem Phys 96(3):2155–2160
Becke AD (1993) J Chem Phys 98(7):5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37(2):785–789
Grimme S (2006) J Comput Chem 27(15):1787–1799
Schäfer A, Horn H, Ahlrichs R (1992) J Chem Phys 97(4):2571–2577
Schäfer A, Huber C, Ahlrichs R (1994) J Chem Phys 100(8):5829–5835
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7(18):3297–3305
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120(1):215–241
Perdew JP (1986) Phys Rev B 33(12):8822–8824
Becke AD (1988) Phys Rev A 38(6):3098–3100
Adamo C, Cossi M, Barone V (1999) J Mol Struc-Theochem 493(1–3):145–157
Ernzerhof M, Scuseria GE (1999) J Chem Phys 110(11):5029–5036
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132(15):154104
Grimme S, Ehrlich S, Goerigk L (2011) J Comput Chem 32(7):1456–1465
Swart M (2008) J Chem Theory Comput 4(12):2057–2066
Barone V, Cossi M (1998) J Phys Chem A 102(11):1995–2001
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comput Chem 24(6):669–681
Fukui K (1970) J Phys Chem 74(23):4161–4163
Lu T, Chen F (2012) J Comput Chem 33(5):580–592
Park MJ, Lee J, Suh Y, Kim J, Nam W (2006) J Am Chem Soc 128(8):2630–2634
Kang Y, Li X-X, Cho K-B, Sun W, Xia C, Nam W, Wang Y (2017) J Am Chem Soc 139(22):7444–7447
Shaik S, Hirao H, Kumar D (2007) Acc Chem Res 40(7):532–542
Acknowledgements
The authors acknowledge the financial support received from the National Natural Science Foundation of China (Project Nos. 21003116, 21173211, 21203218 and 21633013) and from the open fund of the State Key Laboratory of Molecular Reaction Dynamics (Project No. SKLMRD-K201715). The authors also gratefully acknowledge the computing resources and time made available by the Supercomputing Center of Cold and Arid Region Environment and the Engineering Research Institute of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
775_2017_1477_MOESM1_ESM.pdf
The mechanistic details are presented in 12 figures and 10 tables. Cartesian coordinates of all involved complexes are also given. (PDF 3776 kb)
Rights and permissions
About this article
Cite this article
Wang, F., Sun, W., Xia, C. et al. DFT studies of the substituent effects of dimethylamino on non-heme active oxidizing species: iron(V)-oxo species or iron(IV)-oxo acetate aminopyridine cation radical species?. J Biol Inorg Chem 22, 987–998 (2017). https://doi.org/10.1007/s00775-017-1477-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-017-1477-9