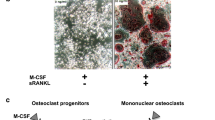
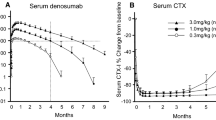
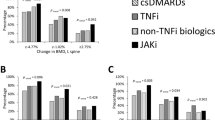
Abstract
Rheumatoid arthritis (RA) is an inflammatory disorder characterized by progressive joint destruction. Recent studies have demonstrated that osteoclasts are responsible for bone destruction in RA. Receptor activator of nuclear factor kappa B ligand (RANKL), an osteoclast differentiation factor, belongs to the tumor necrosis factor superfamily and plays a critical role in osteoclast differentiation. RANKL is highly expressed in the synovial tissues in patients with RA and is involved in osteoclast development and thus bone destruction in RA. Denosumab, a specific antibody to human RANKL, efficiently suppressed the progression of bone destruction in patients with RA in a randomized controlled study and is considered a putative therapeutic option for RA.



Similar content being viewed by others
Change history
17 December 2020
In the original publication of the article, the title of Table was published incorrectly. The correct title should read as “Baseline patient demographics and characteristics in DESIRABLE trial. Adopted from Reference with permission”.
References
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423:356–361
Schett G (2017) Autoimmunity as a trigger for structural bone damage in rheumatoid arthritis. Mod Rheumatol 27:193–197. https://doi.org/10.1080/14397595.2016.1265907
Wassenberg S, Rau R (2002) Radiographic healing with sustained clinical remission in a patient with rheumatoid arthritis receiving methotrexate monotherapy. Arthritis Rheum 46:2804–2807. https://doi.org/10.1002/art.10568
Yamanaka H, Seto Y, Tanaka E, Furuya T, Nakajima A, Ikari K, Taniguchi A, Momohara S (2013) Management of rheumatoid arthritis: the 2012 perspective. Mod Rheumatol 23:1–7. https://doi.org/10.1007/s10165-012-0702-1
Chatzidionysiou K, Emamikia S, Nam J, Ramiro S, Smolen J, van der Heijde D, Dougados M, Bijlsma J, Burmester G, Scholte M, van Vollenhoven R, Landewe R (2017) Efficacy of glucocorticoids, conventional and targeted synthetic disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 76:1102–1107. https://doi.org/10.1136/annrheumdis-2016-210711
Nam JL, Takase-Minegishi K, Ramiro S, Chatzidionysiou K, Smolen JS, van der Heijde D, Bijlsma JW, Burmester GR, Dougados M, Scholte-Voshaar M, van Vollenhoven R, Landewe R (2017) Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 76:1113–1136. https://doi.org/10.1136/annrheumdis-2016-210713
Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K et al (2017) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 76:960–977. https://doi.org/10.1136/annrheumdis-2016-210715
Burmester GR, Pope JE (2017) Novel treatment strategies in rheumatoid arthritis. Lancet 389:2338–2348. https://doi.org/10.1016/S0140-6736(17)31491-5
Ramiro S, Sepriano A, Chatzidionysiou K, Nam JL, Smolen JS, van der Heijde D, Dougados M, van Vollenhoven R, Bijlsma JW, Burmester GR, Scholte-Voshaar M, Falzon L, Landewe RBM (2017) Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 76:1101–1136. https://doi.org/10.1136/annrheumdis-2016-210708
Komano Y, Tanaka M, Nanki T, Koike R, Sakai R et al (2011) Incidence and risk factors for serious infection in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a report from the Registry of Japanese Rheumatoid Arthritis Patients for longterm safety. J Rheumatol 38:1258–1264. https://doi.org/10.3899/jrheum.101009
Tateishi H (1973) Ultrastructure of synovio-cartilage junction in rheumatoid arthritis. Kobe J Med Sci 19:51–66
Bromley M, Woolley DE (1984) Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum 27:968–975
Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR (1998) Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 152:943–951
Suzuki Y, Nishikaku F, Nakatuka M, Koga Y (1998) Osteoclast-like cells in murine collagen induced arthritis. J Rheumatol 25:1154–1160
Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH (2013) Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol 9:522–536. https://doi.org/10.1038/nrendo.2013.137
Kadono Y, Tanaka S, Nishino J, Nishimura K, Nakamura I, Miyazaki T, Takayanagi H, Nakamura K (2009) Rheumatoid arthritis associated with osteopetrosis. Mod Rheumatol 19:687–690. https://doi.org/10.1007/s10165-009-0208-7
Ralston SH, Hacking L, Willocks L, Bruce F, Pitkeathly DA (1989) Clinical, biochemical, and radiographic effects of aminohydroxypropylidene bisphosphonate treatment in rheumatoid arthritis. Ann Rheum Dis 48:396–399
Eggelmeijer F, Papapoulos SE, van Paassen HC, Dijkmans BA, Breedveld FC (1994) Clinical and biochemical response to single infusion of pamidronate in patients with active rheumatoid arthritis: a double blind placebo controlled study. J Rheumatol 21:2016–2020
Herrak P, Gortz B, Hayer S, Redlich K, Reiter E, Gasser J, Bergmeister H, Kollias G, Smolen JS, Schett G (2004) Zoledronic acid protects against local and systemic bone loss in tumor necrosis factor-mediated arthritis (in eng). Arthritis Rheum 50:2327–2337
Sims NA, Green JR, Glatt M, Schlict S, Martin TJ, Gillespie MT, Romas E (2004) Targeting osteoclasts with zoledronic acid prevents bone destruction in collagen-induced arthritis (in eng). Arthritis Rheum 50:2338–2346
Jarrett SJ, Conaghan PG, Sloan VS, Papanastasiou P, Ortmann CE, O'Connor PJ, Grainger AJ, Emery P (2006) Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum 54:1410–1414. https://doi.org/10.1002/art.21824
Fujikawa Y, Sabokbar A, Neale S, Athanasou NA (1996) Human osteoclast formation and bone resorption by monocytes and synovial macrophages in rheumatoid arthritis. Ann Rheum Dis 55:816–822
Takayanagi H, Oda H, Yamamoto S, Kawaguchi H, Tanaka S, Nishikawa T, Koshihara Y (1997) A new mechanism of bone destruction in rheumatoid arthritis: synovial fibroblasts induce osteoclastogenesis. Biochem Biophys Res Commun 240:279–286. https://doi.org/10.1006/bbrc.1997.7404
Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, Goldring SR (2000) Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum 43:250–258
Shigeyama Y, Pap T, Kunzler P, Simmen BR, Gay RE, Gay S (2000) Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum 43:2523–2530
Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S (2000) Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 43:259–269
Takayanagi H, Kim S, Taniguchi T (2002) Signaling crosstalk between RANKL and interferons in osteoclast differentiation. Arthritis Res 4(Suppl 3):S227–S232. https://doi.org/10.1186/ar581
Baron R (1989) Polarity and membrane transport in osteoclasts. Connect Tissue Res 20:109–120
Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H (2006) Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203:2673–2682. https://doi.org/10.1084/jem.20061775
Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H (2014) Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 20:62–68. https://doi.org/10.1038/nm.3432
Danks L, Komatsu N, Guerrini MM, Sawa S, Armaka M, Kollias G, Nakashima T, Takayanagi H (2016) RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis 75:1187–1195. https://doi.org/10.1136/annrheumdis-2014-207137
Hashizume M, Hayakawa N, Mihara M (2008) IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 47:1635–1640. https://doi.org/10.1093/rheumatology/ken363
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A et al (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402:304–309
Pettit AR, Ji H, von Stechow D, Muller R, Goldring SR, Choi Y, Benoist C, Gravallese EM (2001) TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis (in eng). Am J Pathol 159:1689–1699
Redlich K, Hayer S, Ricci R, David JP, Tohidast-Akrad M, Kollias G, Steiner G, Smolen JS, Wagner EF, Schett G (2002) Osteoclasts are essential for TNF-alpha-mediated joint destruction (in eng). J Clin Invest 110:1419–1427
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C, Trial F (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765. https://doi.org/10.1056/NEJMoa0809493
Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, Jiang Q, Tadros S, Dansey R, Goessl C (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377:813–822. https://doi.org/10.1016/S0140-6736(10)62344-6
Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A (2010) Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 28:5132–5139. https://doi.org/10.1200/JCO.2010.29.7101
Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A, Vadhan-Raj S, von Moos R, Willenbacher W, Woll PJ, Wang J, Jiang Q, Jun S, Dansey R, Yeh H (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29:1125–1132. https://doi.org/10.1200/JCO.2010.31.3304
Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, van der Heijde D, Zhou L, Tsuji W, Newmark R, Denosumab Rheumatoid Arthritis Study G (2008) Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum 58:1299–1309. https://doi.org/10.1002/art.23417
Deodhar A, Dore RK, Mandel D, Schechtman J, Shergy W, Trapp R, Ory PA, Peterfy CG, Fuerst T, Wang H, Zhou L, Tsuji W, Newmark R (2010) Denosumab-mediated increase in hand bone mineral density associated with decreased progression of bone erosion in rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 62:569–574. https://doi.org/10.1002/acr.20004
Dore RK, Cohen SB, Lane NE, Palmer W, Shergy W, Zhou L, Wang H, Tsuji W, Newmark R, Denosumab RASG (2010) Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis 69:872–875. https://doi.org/10.1136/ard.2009.112920
Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka H, Yoneda T, Ohira T, Okubo N, Genant HK, van der Heijde D (2016) Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12 month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis 75:983–990. https://doi.org/10.1136/annrheumdis-2015-208052
Takeuchi T, Tanaka Y, Soen S, Yamanaka H, Yoneda T, Tanaka S, Nitta T, Okubo N, Genant HK, van der Heijde D (2019) Effects of the anti-RANKL antibody denosumab on joint structural damage in patients with rheumatoid arthritis treated with conventional synthetic disease-modifying antirheumatic drugs (DESIRABLE study): a randomised, double-blind, placebo-controlled phase 3 trial. Ann Rheum Dis 78:899–907. https://doi.org/10.1136/annrheumdis-2018-214827
Hasegawa T, Kaneko Y, Izumi K, Takeuchi T (2017) Efficacy of denosumab combined with bDMARDs on radiographic progression in rheumatoid arthritis. Joint Bone Spine 84:379–380. https://doi.org/10.1016/j.jbspin.2016.05.010
Tanaka S, Tanaka Y, Ishiguro N, Yamanaka H, Takeuchi T (2018) RANKL: a therapeutic target for bone destruction in rheumatoid arthritis. Mod Rheumatol 28:9–16. https://doi.org/10.1080/14397595.2017.1369491
van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C (2006) Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum 54:3104–3112. https://doi.org/10.1002/art.22117
Brennan SL, Toomey L, Kotowicz MA, Henry MJ, Griffiths H, Pasco JA (2014) Rheumatoid arthritis and incident fracture in women: a case-control study. BMC Musculoskelet Disord 15:13. https://doi.org/10.1186/1471-2474-15-13
Omata Y, Hagiwara F, Nishino J, Matsudaira K, Kadono Y, Juji T, Mori T, Nakayama H, Nagase Y, Hirose J, Yasui T, Matsumoto T, Matsui T, Tohma S, Tanaka S (2014) Vertebral fractures affect functional status in postmenopausal rheumatoid arthritis patients. J Bone Miner Metab 32:725–731. https://doi.org/10.1007/s00774-013-0552-8
Spector TD, Hall GM, McCloskey EV, Kanis JA (1993) Risk of vertebral fracture in women with rheumatoid arthritis. BMJ 306:558
Ochi K, Inoue E, Furuya T, Ikari K, Toyama Y, Taniguchi A, Yamanaka H, Momohara S (2015) Ten-year incidences of self-reported non-vertebral fractures in Japanese patients with rheumatoid arthritis: discrepancy between disease activity control and the incidence of non-vertebral fracture. Osteoporos Int 26:961–968. https://doi.org/10.1007/s00198-014-2911-2
Kim SY, Schneeweiss S, Liu J, Solomon DH (2012) Effects of disease-modifying antirheumatic drugs on nonvertebral fracture risk in rheumatoid arthritis: a population-based cohort study. J Bone Miner Res 27:789–796. https://doi.org/10.1002/jbmr.1489
Mochizuki T, Yano K, Ikari K, Kawakami K, Hiroshima R, Koenuma N, Ishibashi M, Momohara S (2017) Effects of denosumab treatment on bone mineral density and joint destruction in patients with rheumatoid arthritis. J Bone Miner Metab. https://doi.org/10.1007/s00774-017-0848-1
Kinoshita H, Miyakoshi N, Kashiwagura T, Kasukawa Y, Sugimura Y, Shimada Y (2016) Comparison of the efficacy of denosumab and bisphosphonates for treating secondary osteoporosis in patients with rheumatoid arthritis. Mod Rheumatol. https://doi.org/10.1080/14397595.2016.1232776
Saag KG, Pannacciulli N, Geusens P, Adachi JD, Messina OD, Morales-Torres J, Emkey R, Butler PW, Yin X, Lems WF (2019) Denosumab versus risedronate in glucocorticoid-induced osteoporosis: final results of a twenty-four-month randomized, double-blind, double-dummy trial. Arthritis Rheumatol 71:1174–1184. https://doi.org/10.1002/art.40874
Saag KG, Wagman RB, Geusens P, Adachi JD, Messina OD, Emkey R, Chapurlat R, Wang A, Pannacciulli N, Lems WF (2018) Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 6:445–454. https://doi.org/10.1016/S2213-8587(18)30075-5
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Sakae Tanaka received consulting fees, speaking fees, and/or honoraria from Asahi Kasei Pharma Co., Daiichi-Sankyo Co., Ltd., Mitsubishi-Tanabe Pharma Co., and Teijin Pharma, Ltd. and research grants from Astellas Pharma Inc., Asahi Kasei Pharma Co., AYUMI Pharmaceutical Co., Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo Co., Ltd., Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., Stryker Japan K.K., Taisho Toyama Pharmaceutical Co., Ltd., and Teijin Pharma Ltd. Yoshiya Tanaka received speaking fees and/or honoraria from Daiichi-Sankyo Co. Ltd., Eli Lilly Japan K.K., Novartis, YL Biologics Ltd., Bristol-Myers Squibb Co., Eisai Co. Ltd., Chugai Pharmaceutical Co. Ltd., Abbvie, Astellas Pharma Inc., Pfizer Japan Inc., Sanofi K.K., Asahi Kasei Pharma Inc., GSK, Mitsubishi-Tanabe Pharma Co., Gilead Sciences Inc., Janssen Pharmaceutical K.K., and received research grants from Abbvie, Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co. Ltd., Asahi Kasei Pharma Inc., Eisai Co. Ltd., Takeda Pharmaceutical Co. Ltd., Daiichi-Sankyo Co. Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tanaka, S., Tanaka, Y. RANKL as a therapeutic target of rheumatoid arthritis. J Bone Miner Metab 39, 106–112 (2021). https://doi.org/10.1007/s00774-020-01159-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-020-01159-1