Abstract
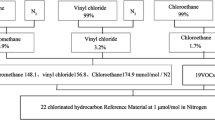
In this study, analytical method validation has been done for the measurement of carbon dioxide/nitrogen (CO2/N2) and methane/nitrogen (CH4/N2) calibration gas mixtures using gas chromatography with flame ionization detector (GC-FID). Class-I calibration gas mixtures (CGMs) of CO2 (500 µmol mol−1 to 1100 µmol mol−1) and CH4 (2 µmol mol−1 to 130 µmol mol−1) used in method validation process has been prepared gravimetrically following ISO 6142-1. All prepared gas mixtures have expanded uncertainty 1 % at coverage factor (k) of 2 with 95 % confidence. The following parameters are chosen for this case study which include selectivity, accuracy, precision, linearity, limit of detection (LOD), limit of quantification (LOQ), robustness, stability, and uncertainty. Different statistical approaches are taken into consideration for each parameter assessment. The results indicate that GC-FID is selective for CO2 and CH4. CGMs represent good repeatability and reproducibility having percentage relative deviation < 1 % among measurements. A good linear behaviour was observed for CGMs of CH4 and CO2 on basis of least square regression with R2 value 0.9995 and 1, respectively. LOD and LOQ for CH4 are calculated 0.47 and 1.59 µmol mol−1 based on signal-to-noise ratio by taking its lowest concentration of 2.9 µmol mol−1. The in-house validated method for GC-FID using CGMs for the measurement of greenhouse gases (CO2 and CH4) is found to be precise, accurate and fit for purpose.







Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author on request.
References
Dai A (2011) Drought under global warming: a review. Wiley Interdiscip Rev Clim Change 2(1):45–65. https://doi.org/10.1002/WCC.81
Emanuel K (2011) Global warming effects on US hurricane damage. Weather Clim Soc 3(4):261–268. https://doi.org/10.1175/WCAS-D-11-00007.1
Perera F (2018) Pollution from Fossil-Fuel Combustion is the Leading Environmental Threat to Global Pediatric Health and Equity: Solutions Exist. Int J Environ Res Public Health. https://doi.org/10.3390/IJERPH15010016
Smith ZA (2017) The environmental policy paradox. https://doi.org/10.4324/9781315623641
Yusuf RO, Noor ZZ, Abba AH, Hassan MAA, Din MFM (2012) Methane emission by sectors: a comprehensive review of emission sources and mitigation methods. Renew Sustain Energy Rev 16(7):5059–5070. https://doi.org/10.1016/J.RSER.2012.04.008
Crosson ER (2008) A cavity ring-down analyzer for measuring atmospheric levels of methane, carbon dioxide, and water vapor. Appl Phys B Lasers Opt. https://doi.org/10.1007/S00340-008-3135-Y
Gavrilov NM, Makarova MV, Poberovskii AV, Timofeyev YM (2014) Comparisons of CH4 ground-based FTIR measurements near Saint Petersburg with GOSAT observations. Atmos Meas Tech 7(4):1003–1010. https://doi.org/10.5194/AMT-7-1003-2014
Kamiński M, Kartanowicz R, Jastrzȩbski D, Kamiński MM (2003) Determination of carbon monoxide, methane and carbon dioxide in refinery hydrogen gases and air by gas chromatography. J Chromatogr A 989(2):277–283. https://doi.org/10.1016/S0021-9673(03)00032-3
Lodge JP (2018) Determination of O2, N2, CO, CO2, and CH4 (Gas Chromatographic Method). Methods Air Sampl Anal. https://doi.org/10.1201/9780203747407-51
Van Der Laan S, Neubert REM, Meijer HAJ (2009) Atmospheric measurement techniques a single gas chromatograph for accurate atmospheric mixing ratio measurements of CO2, CH4, N2O, SF6 and CO. Atmos Meas Tech 2:549–559
Weiss RF (1981) Determinations of carbon dioxide and methane by dual catalyst flame ionization chromatography and nitrous oxide by electron capture chromatography. J Chromatogr Sci 19(12):611–616. https://doi.org/10.1093/CHROMSCI/19.12.611
Hilborn JC, Monkman JL (1975) Gas chromatographic analysis of calibration gas mixtures. Sci Total Environ 4(1):97–106. https://doi.org/10.1016/0048-9697(75)90017-0
International conference on harmonisation of technical requirements ICH harmonised tripartite, Guidelines for validation of analytical procedure (1994)
EURACHEM: The fitness for purpose of analytical methods (2014)
Taverniers I, De Loose M, Van Bockstaele E (2004) Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrAC Trends Anal Chem 23(8):535–552. https://doi.org/10.1016/J.TRAC.2004.04.001
Thompson M, Ellison SLR, Wood R (2002) Resulting from the symposium on harmonization of quality assurance systems for analytical laboratories. Pure Appl Chem 74(5):4–5. https://doi.org/10.1351/pac200274050835
Wenclawiak B, Hadjicostas E (2010) Validation of analytical methods - To be fit for the purpose. Quality Assur Anal Chem Train Teach. https://doi.org/10.1007/978-3-642-13609-2_11/COVER
ISO 6142-1 (2015) Gas analysis—preparation of calibration gas mixtures — Part 1: Gravimetric method for Class I mixtures
CCQM (2019) Mise en pratique - mole - Appendix 2 - SI Brochure
ISO/IEC 17025 (2017) General requirements for the competence of testing and calibration laboratories
Komal, Soni D, Kumari P, Gazal, Singh K, Aggarwal SG (2022) A practical approach of measurement uncertainty evaluation for gravimetrically prepared binary component calibration gas mixture. Mapan J Metrol Soc India, 37(3): 653–664. https://doi.org/10.1007/s12647-022-00600-2
JCGM 100 (2008) Evaluation of measurement data—Guide to the expression of uncertainty in measurement. International Organization for Standardization Geneva
Hong K, Kim BM, Kil Bae H, Lee S, Tshilongo J, Mogale D, Seemane P, Mphamo T, Kadir HA, Ahmad MF, Hidaya N, Nasir A, Baharom N, Soni D, Singh K, Bhat S, Aggarwal SG, Johri P, Kiryong H (2020) Final report international comparison APMP.QM-S7.1 Methane in nitrogen at 2000 μmol/mol. Metrologia. https://doi.org/10.1088/0026-1394/52/1A/08013
Lee J, Lim J, Moon D, Aggarwal SG, Johri P, Soni D, Hui L, Ming KF, Sinweeruthai R, Rattanasombat S, Zuas O, Budiman H, Mulyana MR, Alexandrov V (2021) Final report for supplementary comparison APMP.QM-S15: carbon dioxide in nitrogen at 1000 µmol/mol. Metrologia. https://doi.org/10.1088/0026-1394/58/1A/08014
Ribani M (2004) validation for chromatographic and electrophoretic methods. Quim Nova 27(5):771–780. https://doi.org/10.1590/S0100-40422004000500017
Persson B (2001) The use of selectivity in analytical chemistry. Trends Anla Chem 20(10):526–532. https://doi.org/10.1016/S0165-9936(01)00093-0
Freeman RR, Kukla D (1986) The Role of Selectivity in Gas Chromatography. J Chromatogr Sci 24(9):392–395. https://doi.org/10.1093/CHROMSCI/24.9.392
Foley JP (1991) Resolution equations for column chromatography. Analyst 116(12):1275–1279. https://doi.org/10.1039/AN9911601275
Juradao JM (2017) Some practical considerations for linearity assessment of calibration curves as function of concentration levels according to the fitness for purpose approach. Talanta. https://doi.org/10.1016/j.talanta.2017.05.049
Dorschel CA, Ekmanis JL, Oberholtzer JE, Vincent Warren F, Bidlingmeyer BA (1989) LC detectors: evaluation and practical implications of linearity. Anal Chem 61(17):951A-968A. https://doi.org/10.1021/AC00192A719
Roddam AW (2005) Statistics for the Quality Control Chemistry Laboratory. J R Stat Soc A Stat Soc 168(2):464–464. https://doi.org/10.1111/J.1467-985X.2005.358_13.X
Andrade JM, Gómez-Carracedo MP (2013) Notes on the use of Mandel’s test to check for nonlinearity in laboratory calibrations. Anal Methods 5(5):1145–1149. https://doi.org/10.1039/c2ay26400
Miller JN (1991) Basic statistical methods for analytical chemistry. Part 2. Calibration and regression methods. A review. The Anal 116(1):3–14. https://doi.org/10.1039/AN9911600003
Raposo F (2016) Evaluation of analytical calibration based on least-squares linear regression for instrumental techniques: a tutorial review. TrAC Trends Anal Chem 77:167–185. https://doi.org/10.1016/j.trac.2015.12.006
Rodríguez LC, Campaña AMG, Linares CJ, Ceba MR (1993) Estimation of performance characteristics of an analytical method using the data set of the calibration experiment. Anal Lett 26(6):1243–1258. https://doi.org/10.1080/00032719308019900
Montgomery D, Peck EA, Vining GG (2006) Introduction to linear regression analysis, 4th edn. John Wiley & Sons, New Jersey
Mactaggart DL, Farwell SO (1992) Analytical use of linear regression. Part I: regression procedures for calibration and quantitation. J AOAC Int 75(4):594–608. https://doi.org/10.1093/JAOAC/75.4.594
ISO 5725-1 (2023) Accuracy (trueness and precision) of measurement methods and results—Part 1
ISO 6143 (2001) Gas Analysis—Comparison methods for determining and checking the composition of calibration gas mixture
González AG, Herrador MÁ, Asuero AG (2010) Intra-laboratory assessment of method accuracy (trueness and precision) by using validation standards. Talanta 82(5):1995–1998. https://doi.org/10.1016/J.TALANTA.2010.07.071
Albert R, Horwitz W (1997) A heuristic derivation of the horwitz curve. Anal Chem 69(4):789–790. https://doi.org/10.1021/AC9608376
Horwitz W, Albert R (2006) The Horwitz ratio (HorRat): a useful index of method performance with respect to precision. J AOAC Int 89(4):1095–1109. https://doi.org/10.1093/jaoac/89.4.1095
Shrivastava A, Gupta V (2011) Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron Young Sci 2(1):21. https://doi.org/10.4103/2229-5186.79345
Desimoni E, Brunetti B (2015) About estimating the limit of detection by the signal to noise approach. Pharm Anal Acta. https://doi.org/10.4172/2153-2435.1000355
ISO/IEC Guide 99 (2007) International vocabulary of metrology- Basic and general concepts and associated terms (VIM)
Konieczka P, Namieśnik J (2010) Estimating uncertainty in analytical procedures based on chromatographic techniques. J Chromatogr A 1217(6):882–891. https://doi.org/10.1016/j.chroma.2009.03.078
Satterthwaite FE (1946) An approximate distribution of estimates of variance components. Int Biom Soc 2:110–114. https://doi.org/10.2307/3002019
Welch BL (1947) The generalisation of ‘students’ problem when several different population variances are involved. Biom Bull. https://doi.org/10.1093/biomet/34.1-2.28
ISO Guide 35 (2017) Reference materials—Guidance for characterization and assessment of homogeneity and stability
Acknowledgements
The author, Komal is thankful to Council of Scientific and Industrial Research (CSIR) for providing the fellowship under CSIR-SRF scheme (P- 81-101). Authors are thankful to the Director, CSIR-NPL for providing all support to carry out gas metrology work and further extend their thanks to Head of ESBM Division and Gas Metrology group members for their help and support.
Author information
Authors and Affiliations
Contributions
Ms. Komal did all the experimental analysis, data curation and writing manuscript. Dr Daya Soni supervised in all experimental part , constructing the manuscript and data processing. Dr. Shankar G. Aggarwal reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Komal, Soni, D. & Aggarwal, S.G. A case study for in-house method validation of gas chromatography technique using class-1 calibration gas mixtures for greenhouse gases monitoring. Accred Qual Assur 28, 209–220 (2023). https://doi.org/10.1007/s00769-023-01552-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-023-01552-z