Abstract
Alzheimer’s disease (AD) is a multifactorial neurodegenerative disease that lacks convenient and accessible peripheral blood diagnostic markers and effective drugs. Metabolic dysfunction is one of AD risk factors, which leaded to alterations of various metabolites in the body. Pathological changes of the brain can be reflected in blood metabolites that are expected to explain the disease mechanisms or be candidate biomarkers. The aim of this study was to investigate the changes of targeted metabolites within peripheral blood of AD mouse model, with the purpose of exploring the disease mechanism and potential biomarkers. Targeted metabolomics was used to quantify 256 metabolites in serum of triple transgenic AD (3 × Tg-AD) male mice. Compared with controls, 49 differential metabolites represented dysregulation in purine, pyrimidine, tryptophan, cysteine and methionine and glycerophospholipid metabolism. Among them, adenosine, serotonin, N-acetyl-5-hydroxytryptamine, and acetylcholine play a key role in regulating neural transmitter network. The alteration of S-adenosine-l-homocysteine, S-adenosine-l-methionine, and trimethylamine-N-oxide in AD mice serum can served as indicator of AD risk. The results revealed the changes of metabolites in serum, suggesting that metabolic dysregulation in periphery in AD mice may be related to the disturbances in neuroinhibition, the serotonergic system, sleep function, the cholinergic system, and the gut microbiota. This study provides novel insights into the dysregulation of several key metabolites and metabolic pathways in AD, presenting potential avenues for future research and the development of peripheral biomarkers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease that is a serious health risk for the elderly (Niedowicz et al. 2014; Lang et al. 2012). It is characterized clinically by memory impairment, aphasia, apraxia, agnosia, impairment of visuospatial skills, executive dysfunction, and personality and behavioral changes, and the etiology is still unknown (Conejero-Goldberg et al. 2008). The main pathological features are senile plaque formed by β-amyloid (Aβ) deposits and neurogenic fibrillary tangles (NFT) formed by abnormal aggregation of tau proteins (Abe et al. 2020). The critical factors of AD pathogenesis included genetics, aging, environmental factor, lifestyle habits, viral and bacterial infections, sleep, and the potential role of the microbiota (Guo et al. 2020). The presence of Aβ, tau proteins, as well as glia significantly contribute to the pathogenesis of AD. These factors can disrupt synaptic plasticity, impair synaptic function, disturb intracellular Ca2+ homeostasis, trigger inflammatory-immune responses, induce mitochondrial damage, generate oxidative stress, and cause abnormal lipid and energy metabolism (Pang et al. 2022; Parodi-Rullán et al. 2021; Yu et al. 2009; Forman et al. 2005; Shen et al. 2016). Ultimately, the cumulative effects of these processes result in neuronal apoptosis and dysregulation, and the manifestation of disease. Because of its complex etiology, long incubation period and slow progress, the disease has a 15–20-year evolution process (Diniz-Filho et al. 2017). Early detection and intervention are the main measures in the management of AD. Before the lesion is too serious to be cured, it is essential to make early diagnosis of AD and intervene with medication (Gurevich et al. 2017; Yu et al. 2021; Calvini et al. 2009).
At present, clinical diagnostic methods for AD include psychiatric cognitive scale tests, neuroimaging, and biomarker tests (Shin et al. 2021; Silvestro et al. 2019). However, diagnoses on scales are subjective, and early imaging is focused on positron emission tomography (PET), which is expensive (Lim et al. 2019; Park et al. 2019). Biomarker testing based on cerebrospinal fluid (CSF), and collection requires lumbar puncture is not conducive to screen biomarker (Shin et al. 2021; Zhao et al. 2021a). Blood-based biomarker research has become a current hotspot but it has limited clinical application (Cheng et al. 2020; Shen et al. 2019). The main reason is that the levels of commonly used blood diagnostic markers, such as those associated with Aβ and tau, are extremely low, and existing methods are still unable to meet the requirements of routine detection in terms of sensitivity or detection cost (Mielke et al. 2017; Shen et al. 2019). Additionally, since the pathogenesis of AD is multidimensional and has different pathological characteristics, which is also considered as a metabolic network failure disease (Kaddurah-Daouk et al. 2013; Kang et al. 2017; Toledo et al. 2017). Serum metabolites are expected to distinguish healthy controls and moderate cognitive impairment groups (Zhang et al. 2021b; Toledo et al. 2017) or AD groups (Huo et al. 2020). Therefore, there is an urgent need to conduct biomarker research from different perspectives, explore novel specific early diagnostic markers, and establish highly sensitive and cost-controllable detection methods to achieve early detection and intervention (Shen et al. 2019). Moreover, the specific treatment for AD remains difficult to achieve. Consequently, it is also an urgent need to further clarify the mechanism and search for effective new drug targets.
High-throughput omics approaches offer technical advantages in exploring disease mechanism. Metabolomics provides a powerful and potential method for finding diagnostic markers and new therapeutic targets for AD. In recent years, it has been applied to the research of AD (Xie et al. 2021; Hao et al. 2018; Varma et al. 2018; Yin et al. 2023). As expected, an increasing number of blood metabolites in AD have been successfully identified that are associated with inflammation, oxidative stress, mitochondrial dysfunction, and neuronal damage (Snyder et al. 2014; González-Domínguez et al. 2014; Jia et al. 2022). Interestingly, Aβ was found to be associated with different peripheral metabolites such as bile acid, uric acid, homocysteine, cholesterol and cortisol (Nho et al. 2019; Kiddle et al. 2012; Oxenkrug et al. 2017; Hu et al. 2020; Sun et al. 2020). Abnormalities in serum phosphatidylcholine may reflect disruption of brain membrane integrity (Nho et al. 2021). Lower serum serotonin levels are associated with cognitive decline in AD patients, while higher levels of triacyl carnitine indicate metabolism with long-chain fatty acids for energy supply (Whiley et al. 2021; Huo et al. 2020). In addition, 11 metabolites involved in different metabolic pathways were identified as biomarkers that can distinguished AD from control groups (Sun et al. 2020). A study from 11 cohorts identified subcomponents of high-density lipoprotein (HDL), docosahexaenoic acid (DHA), ornithine, glutamine, and glycoprotein acetyl as biomarkers (van der Lee et al. 2018). Moreover, several metabolites including arachidonic acid, N, N-dimethylglycine, thymine, glutamine, glutamic acid, and cytidine were identified to distinguish AD patients from normal controls (Wang et al. 2014). Therefore, these findings support that those changes in peripheral blood metabolites can indeed reflect the pathophysiological mechanism of AD, and these metabolites have the potential to be served as diagnostic markers. However, more studies are needed to address the specificity and repeatability of metabolic biomarkers, as well as more in-depth studies from a metabolomic perspective.
Targeted metabolomics has significant quantification advantages over non-targeted metabolomics (Zhou and Yin 2016; Lin et al. 2022). In contrast to single detection of a metabolite, a widely targeted metabolic profile in the blood can accurately reflect interrelated biochemical pathway perturbations and identify more effective biomarkers (Venkataraman et al. 2022; Leyane et al. 2022). For example, a targeted metabolomic study showed that three serum acylcarnitines negatively predicted the risk of AD events and cognitive decline from antemortem blood and postmortem brain (Huo et al. 2020). The triple transgenic AD mouse model (3 × Tg-AD) showed the simultaneous existence of Aβ plaques and NFT formation, which mimics the characteristic pathology of human AD, and is an ideal and commonly used mouse model for AD research (Bai et al. 2020; Oddo et al. 2003a; Kim et al. 2018). The aged mice treated with plasma from young wild-type mouse exhibited an improvement of amyloid pathology in brain indicating an interplay between the brain and blood (Zhao et al. 2020). The study of metabolites in peripheral blood can provide clues to explain changes in the brain. In our previous study, we conducted a targeted metabolomic study on 200 metabolites in their hippocampus and eyes (Shen et al. 2023). The results showed significant differences in metabolites between 6-month-old 3 × Tg-AD mice and control mice (Zhao et al. 2021b). In this study, the number of target metabolites was increased to 530. We performed a targeted metabolomics in serum from 6-month-old 3 × Tg-AD mice and control group and aimed to explore the underlying mechanism and potential biomarkers. Moreover, the dysregulated metabolites can contribute some information for the diagnosis and treatment of AD.
Materials and method
Animal and serum samples collection
The original breeding pairs of 3 × Tg-AD mice (Oddo et al. 2003b) harboring human PS1M146V, human APPswe, and human tauP301L and wild-type (WT) control mice (B6:129SF2/J) were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). The mice used in experiment were born in our laboratory. Both 3 × Tg-AD and WT mice were maintained in controlled conditions (12 h light/12 h dark cycle, temperature 22 °C, humidity 50–60%, fresh food, and water ad libitum). In this study, 6-month-old WT male mice (n = 8) and 6-month-old AD male mice (n = 8) were assigned to WT and 3 × Tg-AD group, respectively. All protocols were strictly observed for animal care and welfare according to the NIH guidelines (NIH publication No. 85–23, revised 1985). The study was approved by the Animal Ethical and Welfare Committee of Shenzhen University (Permit Number: AEWC-20140615-002).
At the age of 6 months, the mice were anesthetized with isoflurane (3% initial dose, 1.5% maintenance dose). Blood samples were collected from anesthetized animals by cardiac puncture within disposable sterilized syringe at 9:00 a.m. Preoperative blood (0.6–1.0 mL) was collected in the Eppendorf tube without additives through cardiac puncture. The blood was coagulated at room temperature for 60 min and then centrifuged at 3600 × g for 10 min. The supernatant, serum, was transferred to new Eppendorf tubes and centrifuged at 6500 × g for 3 min. The serum sample (100–200 µL) was stored at − 80 °C until use.
Metabolites’ extraction from serum
The 100 μL of serum sample was diluted by 100 μL ddH2O and mingled with ice-cold extraction buffer (800 μL, methanol/acetonitrile, 1/1, v/v, containing internal standard (IS)) (Table S1). After vortex, the sample was sonicated for 5 min in ice-water bath and incubated in − 40 °C for 2 h. Then, the sample was centrifugated at 12,000 rpm for 15 min at 4 ℃. The 800 μL supernatant was transferred into fresh tube and dried in a vacuum concentrator. The dried samples were reconstituted in 60% acetonitrile by sonication on ice for 10 min. The reconstituted samples were centrifugated at 12,000 rpm for 15 min at 4 ℃ and supernatant was transferred to a fresh glass vial for liquid chromatography-mass spectrometry (LC/MS) analysis.
Targeted LC–MS analysis
Quality control (QC) samples were collected from a pooled sample of all serum samples and used for data normalization. Blank samples (75% ACN in water) and QC samples were injected every eight samples during acquisition. The highest concentration of IS mixture is set to S1, S1/2 for the next level, and dilute step by step to make the standard curve. When the relative standard deviation of IS response is less than 15%, the system is considered stable. The internal standard method was used to quantify targeted metabolites. The ratio of the standard and IS was utilized to rectify instrument fluctuation. The establishment of a database containing specific ions of targeted metabolites was accomplished through the standards and the Human Metabolome Database (HMDB, https://hmdb.ca). The chromatography separation was carried out using a H-Class (Waters, USA) ultra-high-performance liquid chromatography, equipped with a Waters Atlantis Premier BEH Z-HILIC Column (1.7 µm, 2.1 × 150 mm). The flow rate was 0.5 mL/min, and the sample injection volume was 6 μL. The mobile phase consisted of 10 mM ammonium acetate in 20% acetonitrile (8:2 = water: acetonitrile) (pH = 9) (A) and 90% acetonitrile (9:1 = acetonitrile: water) (B) (pH = 9). The linear gradient was set as follows: 0–1 min: 1% B, 1–8 min: 1% B to 100% B, 8–10 min: 100% B, 10–10.1 min: 100% B to 1% B, 10.1–12 min: 1% B. The column temperature was 25 ℃. The auto-sampler temperature was 8 ℃. A SCIEX 6500 triple quadrupole mass spectrometer (AB SCIEX, USA) equipped with IonDrive Turbo V ESI ion source was applied for assay development. Curtain Gas = 35 psi, IonSpray Voltage = + 5000 V/−4500 V, Temperature = 400 °C, Ion Source Gas 1 = 50 psi, Ion Source Gas 2 = 50 psi. The scan type was selected as multiple response monitoring (MRM) to detect the specific parent ion and daughter ion in samples and identify them by database. Duration = 22 min, delay time = 0, cycles = 2640, cycle = 0.5 s.
Quantitative data process
The raw data consisted of 2 quality control and 16 experimental samples, with total detection of 530 targeted metabolites (Table S2). The MS raw data files were converted to the mzXML format by ProteoWizard (RRID: SCR_012056). Subsequently, data were processed by R package XCMS (version 3.2) (RRID: SCR_015538) to generate a data matrix that consisted of the retention time (RT), mass-to-charge ratio (m/z) values, and peak intensity. Metabolic features detected less than 80% in all the QC samples were discarded. The missing values in the original data were simulated, which was the half minimum method to fill. The final concentration (CF, μmol/L) of sample was calculated by means of calculated concentration (CC, μmol/L) multiplied by dilution factor (Dil). The targeted metabolite concentration (CM, nmol/L) was equal to the CF multiplied by the final volume (VF) and the concentration coefficient in the process of pretreatment, divided by the sampling volume V (nmol/L).
Metabolomics data analysis
The metabolomics data analysis was conducted with R (https://www.r-project.org) (RRID: SCR_001905) and online versions of MetaboAnalyst (http://www.metaboanalyst.ca) (RRID: SCR_015539) as described previously (Zhao et al. 2021b; Zhang et al. 2021a). Orthogonal partial least-squares discrimination analysis (OPLS-DA), permutation test, and principal component analysis (PCA) were performed using the SIMCA software (v14.1, Sartorius Stedim Data Analytics AB, Umea, Sweden) (RRID: SCR_014688). The differential expression metabolites (DEMs) were conducted using the R package Limma (v3.48.3) (RRID:SCR_010943) (Ritchie et al. 2015) and variable importance in projection (VIP) scores from OPLS-DA analysis. The cut off values of 1.2-fold change (FC) for up-regulated and down-regulated, p-value < 0.05 and VIP > 1 were set for significantly differential metabolites in two groups after Benjamin–Hochberg correct. The differential metabolites were selected as candidate to perform enrichment analysis. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed in MetaboAnalyst and the pathways were considered significantly in line with impact > 0 and p < 0.05 after Benjamin–Hochberg correct. Metabolite set enrichment analysis (MSEA) based on KEGG and The Small Molecule Pathway Database (SMPDB) were performed using the online tool MetaboAnalyst and the pathways were significant in line with p < 0.05. The heatmaps were drawn using the R package pheatmap (v1.0.12) (RRID:SCR_016418). The order statistics was calculated in the R package robust ranked aggregation (v1.1) (Kolde et al. 2012) and the pathways were significant in line with score < 0.05. The Spearman’s two matrix correlation analysis was estimated in the R package psych (v2.2.3) (RRID: SCR_021744) after Benjamin–Hochberg correct (p < 0.05). The graphical visualization tool was ggplot2 (v3.3.5) (RRID:SCR_014601).
Results
Metabolic profiling to distinguish the 3 × Tg-AD and WT mice serum
By detecting 530 metabolites from 3 × Tg-AD and WT mice serum, a total of 256 targeted metabolites were identified and quantified (Table S3). They were mainly involved in aminoacyl-tRNA biosynthesis, arginine biosynthesis, glycine, serine and threonine metabolism, alanine, aspartate and glutamate metabolism, purine metabolism, pyrimidine metabolism, pantothenate and CoA biosynthesis, tryptophan metabolism, cysteine and methionine metabolism, glycerophospholipid metabolism, and arginine and proline metabolism (Fig. S1).
Multivariate statistical analyses were used to assess separation between groups. The results of PCA showed significant differences between 3 × Tg-AD and WT mice groups (Fig. 1a). The contribution of variables was characterized using the supervised OPLS-DA model, which showed that there were significant differences between metabolites of 3 × Tg-AD and WT mice (Fig. 1b). The model was validated by 200 times permutation test, which showed low Q2 values and a negative intercept of the regression line on the y-axis, indicating that the model was robust and not over-fitted (Fig. 1c). The cluster analysis of 256 targeted metabolites showed the most of 3 × Tg-AD mice could be distinguished in one cluster (Fig. S2).
Identification of differential metabolites in serum
Volcano plot analysis revealed that a total of 49 differential expression metabolites (DEMs) were identified between the 2 groups. Compared with WT mice, 28 metabolites were up-regulated, while 21 metabolites were down-regulated in the serum of AD mice (Fig. 2a, Table 1 and Table S4). These 49 DEMs were used for cluster analysis, and they were able to effectively distinguish AD from WT mice (Fig. 2b). The matchstick plot shows the classification categories of DEMs, which were mainly involved in amino acids, peptides and analogues, nucleosides, nucleotides and analogues, benzenoids, biogenic amines, and organic acids and derivatives (Fig. 2c).
Pathway enrichment analysis and pathway ranking
KEGG pathway analysis showed that these DEMs were primarily and significantly enriched in purine metabolism, cysteine and methionine metabolism, glycerophospholipid metabolism, and tryptophan metabolism (Fig. 3a). SMPDB analysis revealed that more pathways were significantly enriched, including phosphatidylcholine biosynthesis, tyrosine metabolism, betaine metabolism, purine metabolism, and tryptophan pathways (Fig. 3b). To obtain a broad overview of metabolite alterations, we performed MSEA analysis of 256 metabolites in KEGG and SMPDB, respectively. The results showed that the top 3 significantly enriched pathways in KEGG were pyrimidine metabolism, cysteine and methionine metabolism, and purine metabolism (Fig. 3c). In the SMPDB, nicotinate and nicotinamide metabolism, catecholamine biosynthesis, and methionine metabolism ranked in the top 3 (Fig. 3d). Combined with above significant pathway (p < 0.05 and impact > 0) to rank important pathways using order statistics, the results highlighted that pyrimidine metabolism, tryptophan metabolism, and purine metabolism were the priority pathways (Fig. 3e, f, Fig. S3 and Table S5). The DEMs associated with these pathways are listed in Table 2.
The interaction of differential metabolites from purine metabolism
In our results, a majority of DEMs were mainly enriched in purine metabolism pathway. The DEMs in purinergic metabolites were associated with other signaling molecules to form a complex landscape, including adenosine monophosphate (AMP), 5′-Inosinic acid (IMP), adenosine, guanosine-5′-monophosphate (GMP), uric acid, and xanthosine (Fig. 4a). In correlation analysis, we found that there was significant correlation between DEMs in purine metabolism and DEMs in tryptophan metabolism. Serotonin was significantly positively correlated with adenosine, IMP and GMP. In addition, N-acetyl-5-hydroxytryptamine was significantly positively correlated with adenosine, AMP, IMP and GMP and negatively correlated with uric acid (Fig. 4b).
The differential metabolites in purine metabolic pathway and the correlation between metabolites. a The differential metabolites involved in purine metabolic pathway. b Correlation analysis of DEMs in purine and tryptophan metabolism. The white asterisk (*) indicates significant difference (p < 0.05), FC foldchange
Important metabolic pathways and abundance of important differential metabolites between AD and control groups
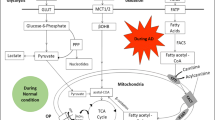
Several important pathways including purine, tryptophan, cysteine and methionine, and glycerophospholipid metabolic pathways and their possible interactions are outlined in Fig. 5a. The concentrations of the 12 important serum differential metabolites between AD and control are shown in Fig. 5b. They were involved in purine metabolism, tryptophan metabolism, cysteine and methionine metabolism, and glycerophospholipid metabolism. Among these, the levels of GMP, adenosine, IMP, serotonin, and CDP-choline were decreased, while acetylcholine, xanthosine, uric acid, trimethylamine-N-oxide (TMAO), N-Acetyl-5-hydroxytryptamine (N-acetyl-5-HT), S-adenosyl-l-methionine (SAM), and S-Adenosyl-l-homocysteine (SAH) were increased in the serum of AD mice.
Summary of several important metabolic pathways. a The reactions within each metabolite are indicated by black arrows. The up-regulated DEMs in pathway are shown in red font. The down-regulated DEMs in pathway are shown in blue font. Metabolites whose concentrations were measured but did not differ are indicated by black text. The gray text indicates the metabolite is not to be detected in serum (Created with BioRender.com.) b Quantification values of DEMs involved in important pathways between 3 × Tg AD and WT mice. The blue background indicates DEMs from purine metabolism. The green background indicates DEMs from tryptophan metabolism. The red background indicates DEMs from cysteine and methionine metabolism. The yellow background indicates DEMs from glycerophospholipid metabolism. *p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, Limma moderated t-test
Discussion
In this study, a targeted metabolomics approach was used to study the changes of metabolites in the serum of 6-month-old 3 × Tg-AD mice. The profiles of these target metabolites showed a significant separation trend between the 3 × Tg-AD and WT mice groups and was able to distinguish the two groups. A total of 49 DEMs were identified between the two groups. Those DEMs were mainly involved in pyrimidine, purine, tryptophan and cysteine and methionine metabolism.
Disturbances in pyrimidine metabolism have recently been frequently associated with AD (Muguruma et al. 2020). In this study, the serum levels of 3-ureidopropionate and thymidine in AD mice were increased, while the levels of cytidine 5′-monophosphate (CMP) were decreased. The increase of 3-ureidopropionate suggests that the degradation of pyrimidine is accelerated (Southern et al. 2015). It acts as an endogenous neurotoxin by inhibiting mitochondrial energy metabolism, leading to secondary, energy-dependent excitotoxicity (Kölker et al. 2001). 3-ureidopropionate has been reported as one of the important factors in AD prediction (Rule et al. 2004).
Neurodegenerative disorders like AD can be attributed to abnormal purinergic signal spread (Huang et al. 2021; Woods et al. 2016). In the present study, upstream DEMs such as adenosine, AMP, IMP and GMP had low concentrations, while downstream DEMs (uric acid and xanthosine) had significantly increased concentrations in the serum of AD mice. Adenosine is an endogenous autacoid with a wide range of roles of inhibitory neuromodulator (Alonso-Andres et al. 2018; Snyder 1985; Merighi et al. 2022; Silva et al. 2018; Liu et al. 2019b). Similarly, its levels in blood were found to be decreased with advancing age and were significantly decreased in an aging and AD mice model (Sanchez-Melgar et al. 2020; Simard et al. 2019). Another recent study showed that it was down-regulated in the hippocampus of AD model mice and increased after drug treatment (Liu et al. 2019a). However, other studies have observed that its levels are increased in the blood of AD patients (Teruya et al. 2021; González-Domínguez et al. 2015) and in the hippocampus of 3 × Tg-AD mice (Zhao et al. 2021b). These discrepancies could potentially arise from the development of AD. Adenosine levels was impacted by serum adenosine deaminase which the activity was changed with aging (Vasudha et al. 2006; Sánchez-Melgar et al. 2020). Additionally, the colocalization of A1 receptors (A1R) with Aβ in senile plaques was observed in post-mortem hippocampal tissue of AD patients and the activity of A1R can meditated tau phosphorylation and translocation (Angulo et al. 2003). Adenosine is a neuroprotective agent that binds to adenosine receptors on cell membranes (particularly A1and A2A adenosine receptors) (Chang et al. 2021). Adenosine and its receptors are potential targets for AD (Liu et al. 2019a). We speculated that adenosine in periphery is associated with the increase of receptor in brain tissue. However, the strength of these links needs further exploration. Uric acid is an endogenous antioxidant and the main end product of purine metabolism (Tan et al. 2016). Some studies have shown that it was down-regulated in the blood of AD patients and associated with poorer cognitive function and an increased risk of MCI or overall dementia (Kim et al. 2020; Mullan et al. 2018; Liu et al. 2017; Euser et al. 2009). However, previous studies have demonstrated that Aβ expressions lead to a shift in purine metabolism, resulting in an increase of uric acid in AD mice (Esteve et al. 2017;Li et al. 2016; Munasinghe et al. 2022). Uric acid may also be associated with increased neuroinflammation in AD (Esteve et al. 2017). One hypothesis proposed that in some cases, particularly during the early stages of disease, although uric acid causes inflammation, it may be increased by antioxidant defenses against oxidation and free radicals (Ames et al. 1981; Bowman et al. 2010; Du et al. 2016). Therefore, AD may be underlined by low-grade chronic inflammation, both at local and peripheral level (Guzman-Martinez et al. 2019). Clearly, this hypothesis needs to be further confirmed.
Moreover, while serotonin is widely accepted to be related to depression, disruption of serotonergic signaling has been shown to be strongly associated with the pathogenesis of AD (Whiley et al. 2021; Smith et al. 2017). In this study, serotonin and N − acetyl − 5 − hydroxytryptamine (N-acetyl-5-HT), which are involved in tryptophan metabolism, were down-regulated in the serum of AD mice. The decrease of serotonin in serum and CSF has been observed, and its receptors were also found decrease in post-mortem brains of AD patient (Whiley et al. 2021; Tohgi et al. 1992; Garcia-Alloza et al. 2005). N-acetyl-5-HT is an intermediate substance between serotonin and melatonin. N-acetyl-5-HT and melatonin showed neuroprotection against amyloid-β peptide aggregation and cytotoxicity (Hornedo-Ortega et al. 2018; Sun et al. 2022; Oxenkrug 2005). Compelling evidence showed that a serotonin degeneration and was associated with an increase in the Aβ-related inflammatory response (Liu et al. 2008; Metaxas et al. 2019). The inflammatory process in AD is characterized by shunted tryptophan metabolism away from serotonin (Willette et al. 2021). The initial degeneration of serotonin may trigger a spread of AD pathology and accumulating studies suggested that serotonin is a potential early hallmarks of AD pathology (Gallo et al. 2021). Together, Aβ deposit may triggered an inflammation with the presence of dysregulated tryptophan metabolism.
Cysteine and methionine metabolic pathway was also a pathway of interest, and SAH and SAM were involved. SAH were decreased, while SAM was increased in the serum of AD mice. They are important precursors in melatonin synthesis. A growing body of research suggests that sleep deprivation is considered as a risk factor for AD. Supplementation of melatonin bring beneficial effect to AD mice and patients (Uddin et al. 2020; Rudnitskaya et al. 2015; Irwin and Vitiello 2019). N-acetyl-5-HT is converted to melatonin by O-methylation of the 5-hydroxy group using S-adenosyl-l-methionine as the methyl donor under the catalysis of hydroxyindole-O-methyltransferase (Itoh et al. 1997). Therefore, the deficiency of N-acetyl-5-HT may reduce the synthesis of melatonin and SAH and contributed to accumulate SAM. Methionine has been demonstrated to alleviate a variety of risk factors and hallmarks associated with AD (Shea and Chan 2008). Methionine has been found to be down-regulated in the hippocampus of 3 × Tg-AD mice in our previous study (Zhao et al. 2021b). An abnormal SAM/SAH ratio is decreased as a character of cognitive decline and death with dementia in elderly populations (Linnebank et al. 2010; Mihara et al. 2022; Guiraud et al. 2017; Mihara et al. 2022). SAM and SAH have been observed to be increased in the brain of AD patients (Mahajan et al. 2020). Another study reported that lower SAH and higher SAM concentrations in the plasma of AD patients (Guiraud et al. 2017). Besides, a previous study proposed that a metabolic link between methionine and phospholipid metabolism may contribute to cerebrovascular and neurodegenerative changes in AD (Obeid and Herrmann 2006).
It is widely known that dysregulation of cholinergic and monoaminergic systems is associated with the pathogenesis of AD (Zong et al. 2022). Acetylcholinesterase (AChE) inhibitor currently is used in AD therapy (Bortolami et al. 2021). In this study, the high levels of acetylcholine were detected in the serum of AD mice. One study observed that acetylcholine was increased in the temporal cortex of AD patients (Kim et al. 2019). On the contrary, lower levels of acetylcholine were observed in plasma of mild cognitive impairment (MCI) (Peña-Bautista et al. 2020), CSF of AD patients (Kumar et al. 1989) and the hippocampus of AD patients (Liu et al. 2021). The dysregulated AChE has a significant impact on availability of acetylcholine (Sadia et al. 2023) and AChE activity progressively diminishes as the severity of dementia advances (Lane et al. 2006). In addition, a previous study speculated that a compensatory system of acetylcholine was activated to reduce Aβ accumulation when the level of serotonin was abnormal in AD (Madsen et al. 2011). Therefore, the compensatory reaction and AChE activity may contribute to the high level of acetylcholine in serum. Another cholinergic precursor, CDP-choline was found to decrease in the serum of 3 × Tg-AD mice. CDP-choline has been shown to elicit advantageous effects in the brain including inhibition of apoptosis, enhancement of neuroplasticity and synthesis of phospholipids and acetylcholine (Gareri et al. 2017; Adibhatla et al. 2002; Fioravanti and Yanagi 2000, 2004).
Furthermore, we identified up-regulation of TMAO levels in 3xTg-AD mice serum. TMAO is a metabolite derived from choline in intestinal flora and have been considered as a risk factor of neurological disorders (Chhibber-Goel et al. 2017; Arrona Cardoza et al. 2022; Buawangpong et al. 2022; Janeiro et al. 2018). The higher levels of TMAO were commonly observed in the plasma, CSF, or brain in both humans or mice (Li et al. 2018; Chen et al. 2022; Vogt et al. 2018; Govindarajulu et al. 2020). Previous studies have shown that peripheral TMAO levels are highly correlated with levels in the brain (Brunt et al. 2021). Elevated serum concentrations of TMAO increased the cellular inflammatory response probably by activating microglia and astrocytes and its perfusion of the brain parenchyma caused progression of small-vessel diseases leading to white matter degeneration observed in AD (Buawangpong et al. 2022; Pietroboni et al. 2020; Brown et al. 2002). These findings support that the disturbance of intestinal flora, along with its metabolites, can reaches central nervous system (CNS), and may, therefore, impairs neurological function (Cryan et al. 2020; Rutsch et al. 2020). Moreover, in this study, serum levels of glyceric acid were reduced in AD mice. Similarly, glyceric acid was down-regulated in the hippocampus of APP/PS1 (AD) mice (Hunsberger et al. 2020) and 3 × Tg-AD mice (Zhao et al. 2021b).
We note that the DEMs identified in this study may interact with one another or converge on a shared mechanism. Adenosine are distributed both presynaptically and postsynaptically in brain leading to inhibit the releases of serotonin and other excitatory neurotransmitter (van Calker et al. 2019; Ribeiro 1995). Here, DEMs from purine pathway (adenosine, AMP, GMP and IMP) and tryptophan pathways (serotonin and N-acetyl-5-HT) suggests showed strong correlation. A novel perspective suggests that gastrointestinal physiology is highly regulated by innervation from the CNS via vagus nerve (Sundman et al. 2017; Mercado et al. 2022; Aaldijk and Vermeiren 2022; El-Merahbi et al. 2015). A study suggested that the accumulation or degradation of adenosine both regulate 5-hydroxytryptamine release from a human enterochromaffin cell through different adenosine receptors (Christofi et al. 2004). The gastrointestinal tract is largest serotonin pool (Yano et al. 2015). Thus, the decline in peripheral serotonin and N-acetyl-5-HT may be caused by dysregulation of neurotransmitters like adenosine. In addition, as mentioned above, serotonin and TMAO are associated with the gut microbiota (Yano et al. 2015; Brunt et al. 2021), supporting that the disturbance of intestinal flora and its metabolites may be associated with the pathogenesis of AD (Cryan et al. 2020; Rutsch et al. 2020). Moreover, compared with the previous study in the hippocampus of 3 × Tg-AD mice (Zhao et al. 2021b), the expression trend of 6 DEMs (3-Ureidopropionate, CMP, adenosine, AMP, IMP and CDP-choline) was reversed. Although the reasons require further investigation, the results suggest that purine and pyrimidine metabolism may play a key role in the pathogenesis of AD.
It is important to note that although we did our best to compare the DEMs and their associated pathways screened in this study with previous studies, their specificity, that is whether they are AD-specific or may represent a broader range of changes associated with aging or different neurodegenerative conditions, needs to be validated by experiments or datasets that include additional animal or human samples, especially human samples.
Conclusion
In the present study, we have carried out a serum-targeted metabolomics study in 6-month-old 3 × Tg-AD mice versus controls and observed broad dysregulation of metabolites. Of these, adenosine may play a role in purine metabolism and interacts with neuromodulator networks. The decrease in serotonin, and alterations in N-acetyl-5-HT and SAM involved in the synthesis of melatonin, suggest a relationship between depression, sleep disorders and AD. Acetylcholine and CDP-choline may act as a compensation to reduce the contraction of nervous system. TMAO and serotonin suggest a link between AD and gut microbiota for metabolites in the serum. A dynamic bidirectional communication system between the gut, microbiome, the blood, and CNS may play a role in AD. Here, the alteration of these metabolic pathways in blood seem to converge on promoting inflammation and were associated with gut microbiota. This study will provide new insights into the therapeutic and diagnostic markers of AD. However, further studies and validation in animals and populations are required.
Data availability
All raw data and corresponding parameter have been deposited as online resource to the Figshare database with the name: “Investigating Metabolic Dysregulation in Serum of Triple Transgenic Alzheimer’s Disease Male Mice: Implications for Pathogenesis and Potential Biomarkers”. (https://doi.org/10.6084/m9.figshare.22731110).
References
Aaldijk E, Vermeiren Y (2022) The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: a narrative review. Ageing Res Rev 75:101556. https://doi.org/10.1016/j.arr.2021.101556
Abe Y, Ikegawa N, Yoshida K, Muramatsu K, Hattori S, Kawai K, Murakami M, Tanaka T, Goda W, Goto M, Yamamoto T, Hashimoto T, Yamada K, Shibata T, Misawa H, Mimura M, Tanaka KF, Miyakawa T, Iwatsubo T, Hata JI, Niikura T, Yasui M (2020) Behavioral and electrophysiological evidence for a neuroprotective role of aquaporin-4 in the 5xFAD transgenic mice model. Acta Neuropathol Commun 8(1):67. https://doi.org/10.1186/s40478-020-00936-3
Adibhatla RM, Hatcher JF, Dempsey RJ (2002) Citicoline: neuroprotective mechanisms in cerebral ischemia. J Neurochem 80(1):12–23. https://doi.org/10.1046/j.0022-3042.2001.00697.x
Alonso-Andres P, Albasanz JL, Ferrer I, Martin M (2018) Purine-related metabolites and their converting enzymes are altered in frontal, parietal and temporal cortex at early stages of Alzheimer’s disease pathology. Brain Pathol 28(6):933–946. https://doi.org/10.1111/bpa.12592
Ames BN, Cathcart R, Schwiers E, Hochstein P (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A 78(11):6858–6862. https://doi.org/10.1073/pnas.78.11.6858
Angulo E, Casadó V, Mallol J, Canela EI, Viñals F, Ferrer I, Lluis C, Franco R (2003) A1 adenosine receptors accumulate in neurodegenerative structures in Alzheimer disease and mediate both amyloid precursor protein processing and tau phosphorylation and translocation. Brain Pathol 13(4):440–451. https://doi.org/10.1111/j.1750-3639.2003.tb00475.x
Arrona Cardoza P, Spillane MB, Morales Marroquin E (2022) Alzheimer’s disease and gut microbiota: does trimethylamine N-oxide (TMAO) play a role? Nutr Rev 80(2):271–281. https://doi.org/10.1093/nutrit/nuab022
Bai B, Wang X, Li Y, Chen PC, Yu K, Dey KK, Yarbro JM, Han X, Lutz BM, Rao S, Jiao Y, Sifford JM, Han J, Wang M, Tan H, Shaw TI, Cho JH, Zhou S, Wang H, Niu M, Mancieri A, Messler KA, Sun X, Wu Z, Pagala V, High AA, Bi W, Zhang H, Chi H, Haroutunian V, Zhang B, Beach TG, Yu G, Peng J (2020) Deep multilayer brain proteomics identifies molecular networks in Alzheimer’s disease progression. Neuron 105(6):975-991.e977. https://doi.org/10.1016/j.neuron.2019.12.015
Bortolami M, Rocco D, Messore A, Di Santo R, Costi R, Madia VN, Scipione L, Pandolfi F (2021) Acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease—a patent review (2016-present). Expert Opin Ther Pat 31(5):399–420. https://doi.org/10.1080/13543776.2021.1874344
Bowman GL, Shannon J, Frei B, Kaye JA, Quinn JF (2010) Uric acid as a CNS antioxidant. J Alzheimers Dis 19(4):1331–1336. https://doi.org/10.3233/jad-2010-1330
Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA (2002) Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci 203–204:159–163. https://doi.org/10.1016/s0022-510x(02)00283-6
Brunt VE, LaRocca TJ, Bazzoni AE, Sapinsley ZJ, Miyamoto-Ditmon J, Gioscia-Ryan RA, Neilson AP, Link CD, Seals DR (2021) The gut microbiome-derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. Geroscience 43(1):377–394. https://doi.org/10.1007/s11357-020-00257-2
Buawangpong N, Pinyopornpanish K, Siri-Angkul N, Chattipakorn N, Chattipakorn SC (2022) The role of trimethylamine-N-Oxide in the development of Alzheimer’s disease. J Cell Physiol 237(3):1661–1685. https://doi.org/10.1002/jcp.30646
Calvini P, Chincarini A, Gemme G, Penco MA, Squarcia S, Nobili F, Rodriguez G, Bellotti R, Catanzariti E, Cerello P, De Mitri I, Fantacci ME (2009) Automatic analysis of medial temporal lobe atrophy from structural MRIs for the early assessment of Alzheimer disease. Med Phys 36(8):3737–3747. https://doi.org/10.1118/1.3171686
Chang CP, Wu KC, Lin CY, Chern Y (2021) Emerging roles of dysregulated adenosine homeostasis in brain disorders with a specific focus on neurodegenerative diseases. J Biomed Sci 28(1):70. https://doi.org/10.1186/s12929-021-00766-y
Chen X, Gu M, Hong Y, Duan R, Zhou J (2022) Association of Trimethylamine N-oxide with normal aging and neurocognitive disorders: a narrative review. Brain Sci 12(9):1203. https://doi.org/10.3390/brainsci12091203
Cheng L, Vella LJ, Barnham KJ, McLean C, Masters CL, Hill AF (2020) Small RNA fingerprinting of Alzheimer’s disease frontal cortex extracellular vesicles and their comparison with peripheral extracellular vesicles. J Extracell Vesicles 9(1):1766822. https://doi.org/10.1080/20013078.2020.1766822
Chhibber-Goel J, Singhal V, Parakh N, Bhargava B, Sharma A (2017) The metabolite Trimethylamine-N-Oxide is an emergent biomarker of human health. Curr Med Chem 24(36):3942–3953. https://doi.org/10.2174/0929867323666160830104025
Christofi FL, Kim M, Wunderlich JE, Xue J, Suntres Z, Cardounel A, Javed NH, Yu JG, Grants I, Cooke HJ (2004) Endogenous adenosine differentially modulates 5-hydroxytryptamine release from a human enterochromaffin cell model. Gastroenterology 127(1):188–202. https://doi.org/10.1053/j.gastro.2004.04.070
Conejero-Goldberg C, Davies P, Ulloa L (2008) Alpha7 nicotinic acetylcholine receptor: a link between inflammation and neurodegeneration. Neurosci Biobehav Rev 32(4):693–706. https://doi.org/10.1016/j.neubiorev.2007.10.007
Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG (2020) The gut microbiome in neurological disorders. Lancet Neurol 19(2):179–194. https://doi.org/10.1016/s1474-4422(19)30356-4
Diniz-Filho A, Delano-Wood L, Daga FB, Cronemberger S, Medeiros FA (2017) Association between neurocognitive decline and visual field variability in glaucoma. JAMA Ophthalmol 135(7):734–739. https://doi.org/10.1001/jamaophthalmol.2017.1279
Du N, Xu D, Hou X, Song X, Liu C, Chen Y, Wang Y, Li X (2016) Inverse association between serum uric acid levels and Alzheimer’s disease risk. Mol Neurobiol 53(4):2594–2599. https://doi.org/10.1007/s12035-015-9271-6
El-Merahbi R, Löffler M, Mayer A, Sumara G (2015) The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett 589(15):1728–1734. https://doi.org/10.1016/j.febslet.2015.05.054
Esteve C, Jones EA, Kell DB, Boutin H, McDonnell LA (2017) Mass spectrometry imaging shows major derangements in neurogranin and in purine metabolism in the triple-knockout 3×Tg Alzheimer mouse model. Biochim Biophys Acta Proteins Proteom 1865(7):747–754. https://doi.org/10.1016/j.bbapap.2017.04.002
Euser SM, Hofman A, Westendorp RG, Breteler MM (2009) Serum uric acid and cognitive function and dementia. Brain 132(Pt 2):377–382. https://doi.org/10.1093/brain/awn316
Forman MS, Lal D, Zhang B, Dabir DV, Swanson E, Lee VM, Trojanowski JQ (2005) Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J Neurosci 25(14):3539–3550. https://doi.org/10.1523/jneurosci.0081-05.2005
Gallo A, Pillet LE, Verpillot R (2021) New frontiers in Alzheimer’s disease diagnostic: monoamines and their derivatives in biological fluids. Exp Gerontol 152:111452. https://doi.org/10.1016/j.exger.2021.111452
Garcia-Alloza M, Gil-Bea FJ, Diez-Ariza M, Chen CP, Francis PT, Lasheras B, Ramirez MJ (2005) Cholinergic-serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer’s disease. Neuropsychologia 43(3):442–449. https://doi.org/10.1016/j.neuropsychologia.2004.06.007
Gareri P, Castagna A, Cotroneo AM, Putignano D, Conforti R, Santamaria F, Marino S, Putignano S (2017) The Citicholinage Study: Citicoline Plus Cholinesterase inhibitors in aged patients affected with Alzheimer’s Disease Study. J Alzheimers Dis 56(2):557–565. https://doi.org/10.3233/jad-160808
González-Domínguez R, García A, García-Barrera T, Barbas C, Gómez-Ariza JL (2014) Metabolomic profiling of serum in the progression of Alzheimer’s disease by capillary electrophoresis-mass spectrometry. Electrophoresis 35(23):3321–3330. https://doi.org/10.1002/elps.201400196
González-Domínguez R, García-Barrera T, Gómez-Ariza JL (2015) Metabolite profiling for the identification of altered metabolic pathways in Alzheimer’s disease. J Pharm Biomed Anal 107:75–81. https://doi.org/10.1016/j.jpba.2014.10.010
Govindarajulu M, Pinky PD, Steinke I, Bloemer J, Ramesh S, Kariharan T, Rella RT, Bhattacharya S, Dhanasekaran M, Suppiramaniam V, Amin RH (2020) Gut metabolite TMAO induces synaptic plasticity deficits by promoting endoplasmic reticulum stress. Front Mol Neurosci 13:138. https://doi.org/10.3389/fnmol.2020.00138
Guiraud SP, Montoliu I, Da Silva L, Dayon L, Galindo AN, Corthésy J, Kussmann M, Martin FP (2017) High-throughput and simultaneous quantitative analysis of homocysteine-methionine cycle metabolites and co-factors in blood plasma and cerebrospinal fluid by isotope dilution LC-MS/MS. Anal Bioanal Chem 409(1):295–305. https://doi.org/10.1007/s00216-016-0003-1
Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y (2020) Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener 15(1):40. https://doi.org/10.1186/s13024-020-00391-7
Gurevich P, Stuke H, Kastrup A, Stuke H, Hildebrandt H (2017) Neuropsychological testing and machine learning distinguish Alzheimer’s disease from other causes for cognitive impairment. Front Aging Neurosci 9:114. https://doi.org/10.3389/fnagi.2017.00114
Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG, Ramos-Escobar N (2019) Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol 10:1008. https://doi.org/10.3389/fphar.2019.01008
Hao L, Wang J, Page D, Asthana S, Zetterberg H, Carlsson C, Okonkwo OC, Li L (2018) Comparative evaluation of MS-based metabolomics software and its application to preclinical Alzheimer’s disease. Sci Rep 8(1):9291. https://doi.org/10.1038/s41598-018-27031-x
Hu N, Gao L, Jiang Y, Wei S, Shang S, Chen C, Dang L, Wang J, Huo K, Deng M, Wang J, Qu Q (2020) The relationship between blood lipids and plasma amyloid beta is depend on blood pressure: a population-based cross-sectional study. Lipids Health Dis 19(1):8. https://doi.org/10.1186/s12944-020-1191-4
Huang Z, Xie N, Illes P, Di Virgilio F, Ulrich H, Semyanov A, Verkhratsky A, Sperlagh B, Yu SG, Huang C, Tang Y (2021) From purines to purinergic signalling: molecular functions and human diseases. Signal Transduct Target Ther 6(1):162. https://doi.org/10.1038/s41392-021-00553-z
Hunsberger HC, Greenwood BP, Tolstikov V, Narain NR, Kiebish MA, Denny CA (2020) Divergence in the metabolome between natural aging and Alzheimer’s disease. Sci Rep 10(1):12171. https://doi.org/10.1038/s41598-020-68739-z
Huo Z, Yu L, Yang J, Zhu Y, Bennett DA, Zhao J (2020) Brain and blood metabolome for Alzheimer’s dementia: findings from a targeted metabolomics analysis. Neurobiol Aging 86:123–133. https://doi.org/10.1016/j.neurobiolaging.2019.10.014
Irwin MR, Vitiello MV (2019) Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol 18(3):296–306. https://doi.org/10.1016/s1474-4422(18)30450-2
Itoh MT, Hattori A, Sumi Y (1997) Hydroxyindole-O-methyltransferase activity assay using high-performance liquid chromatography with fluorometric detection: determination of melatonin enzymatically formed from N-acetylserotonin and S-adenosyl-L-methionine. J Chromatogr B Biomed Sci Appl 692(1):217–221. https://doi.org/10.1016/s0378-4347(96)00503-8
Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M (2018) Implication of Trimethylamine N-Oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients 10(10):1398. https://doi.org/10.3390/nu10101398
Jia L, Yang J, Zhu M, Pang Y, Wang Q, Wei Q, Li Y, Li T, Li F, Wang Q, Li Y, Wei Y (2022) A metabolite panel that differentiates Alzheimer’s disease from other dementia types. Alzheimers Dement 18(7):1345–1356. https://doi.org/10.1002/alz.12484
Kaddurah-Daouk R, Zhu H, Sharma S, Bogdanov M, Rozen SG, Matson W, Oki NO, Motsinger-Reif AA, Churchill E, Lei Z, Appleby D, Kling MA, Trojanowski JQ, Doraiswamy PM, Arnold SE (2013) Alterations in metabolic pathways and networks in Alzheimer’s disease. Transl Psychiatry 3(4):e244. https://doi.org/10.1038/tp.2013.18
Kang S, Lee YH, Lee JE (2017) Metabolism-centric overview of the pathogenesis of Alzheimer’s disease. Yonsei Med J 58(3):479–488. https://doi.org/10.3349/ymj.2017.58.3.479
Kiddle SJ, Thambisetty M, Simmons A, Riddoch-Contreras J, Hye A, Westman E, Pike I, Ward M, Johnston C, Lupton MK, Lunnon K, Soininen H, Kloszewska I, Tsolaki M, Vellas B, Mecocci P, Lovestone S, Newhouse S, Dobson R (2012) Plasma based markers of [11C] PiB-PET brain amyloid burden. PLoS ONE 7(9):e44260. https://doi.org/10.1371/journal.pone.0044260
Kim DK, Park J, Han D, Yang J, Kim A, Woo J, Kim Y, Mook-Jung I (2018) Molecular and functional signatures in a novel Alzheimer’s disease mouse model assessed by quantitative proteomics. Mol Neurodegener 13(1):2. https://doi.org/10.1186/s13024-017-0234-4
Kim YH, Shim HS, Kim KH, Lee J, Chung BC, Kowall NW, Ryu H, Lee J (2019) Metabolomic analysis identifies alterations of amino acid metabolome signatures in the postmortem brain of Alzheimer’s disease. Exp Neurobiol 28(3):376–389. https://doi.org/10.5607/en.2019.28.3.376
Kim JW, Byun MS, Yi D, Lee JH, Jeon SY, Ko K, Jung G, Lee HN, Lee JY, Sohn CH, Lee YS, Shin SA, Kim YK, Lee DY (2020) Serum uric acid, Alzheimer-related brain changes, and cognitive impairment. Front Aging Neurosci 12:160. https://doi.org/10.3389/fnagi.2020.00160
Kolde R, Laur S, Adler P, Vilo J (2012) Robust rank aggregation for gene list integration and meta-analysis. Bioinformatics 28(4):573–580. https://doi.org/10.1093/bioinformatics/btr709
Kölker S, Okun JG, Hörster F, Assmann B, Ahlemeyer B, Kohlmüller D, Exner-Camps S, Mayatepek E, Krieglstein J, Hoffmann GF (2001) 3-Ureidopropionate contributes to the neuropathology of 3-ureidopropionase deficiency and severe propionic aciduria: a hypothesis. J Neurosci Res 66(4):666–673. https://doi.org/10.1002/jnr.10012
Lane RM, Potkin SG, Enz A (2006) Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int J Neuropsychopharmacol 9(1):101–124. https://doi.org/10.1017/S1461145705005833
Lang M, Wang L, Fan Q, Xiao G, Wang X, Zhong Y, Zhou B (2012) Genetic inhibition of solute-linked carrier 39 family transporter 1 ameliorates aβ pathology in a Drosophila model of Alzheimer’s disease. PLoS Genet 8(4):e1002683. https://doi.org/10.1371/journal.pgen.1002683
Leyane TS, Jere SW, Houreld NN (2022) Oxidative stress in ageing and chronic degenerative pathologies: molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int J Mol Sci 23(13):7273. https://doi.org/10.3390/ijms23137273
Li N, Liu Y, Li W, Zhou L, Li Q, Wang X, He P (2016) A UPLC/MS-based metabolomics investigation of the protective effect of ginsenosides Rg1 and Rg2 in mice with Alzheimer’s disease. J Ginseng Res 40(1):9–17. https://doi.org/10.1016/j.jgr.2015.04.006
Li D, Ke Y, Zhan R, Liu C, Zhao M, Zeng A, Shi X, Ji L, Cheng S, Pan B, Zheng L, Hong H (2018) Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 17(4):e12768. https://doi.org/10.1111/acel.12768
Lim CZJ, Zhang Y, Chen Y, Zhao H, Stephenson MC, Ho NRY, Chen Y, Chung J, Reilhac A, Loh TP, Chen CLH, Shao H (2019) Subtyping of circulating exosome-bound amyloid β reflects brain plaque deposition. Nat Commun 10(1):1144. https://doi.org/10.1038/s41467-019-09030-2
Linnebank M, Popp J, Smulders Y, Smith D, Semmler A, Farkas M, Kulic L, Cvetanovska G, Blom H, Stoffel-Wagner B, Kölsch H, Weller M, Jessen F (2010) S-adenosylmethionine is decreased in the cerebrospinal fluid of patients with Alzheimer’s disease. Neurodegener Dis 7(6):373–378. https://doi.org/10.1159/000309657
Liu Y, Yoo MJ, Savonenko A, Stirling W, Price DL, Borchelt DR, Mamounas L, Lyons WE, Blue ME, Lee MK (2008) Amyloid pathology is associated with progressive monoaminergic neurodegeneration in a transgenic mouse model of alzheimer's disease. J Neurosci 28(51):13805–13814. https://doi.org/10.1523/JNEUROSCI.4218-08.2008
Liu M, Wang J, Zeng J, He Y (2017) Relationship between serum uric acid level and mild cognitive impairment in Chinese community elderly. BMC Neurol 17(1):146. https://doi.org/10.1186/s12883-017-0929-8
Liu Y, Du T, Zhang W, Lu W, Peng Z, Huang S, Sun X, Zhu X, Chen C, Qian L, Wen L, Xu P, Zhang Y (2019a) Modified Huang-Lian-Jie-Du decoction ameliorates Aβ synaptotoxicity in a murine model of Alzheimer’s disease. Oxid Med Cell Longev 2019:8340192. https://doi.org/10.1155/2019/8340192
Liu YJ, Chen J, Li X, Zhou X, Hu YM, Chu SF, Peng Y, Chen NH (2019b) Research progress on adenosine in central nervous system diseases. CNS Neurosci Ther 25(9):899–910. https://doi.org/10.1111/cns.13190
Liu P, Yang Q, Yu N, Cao Y, Wang X, Wang Z, Qiu WY, Ma C (2021) Phenylalanine metabolism is dysregulated in human hippocampus with Alzheimer’s disease related pathological changes. J Alzheimers Dis 83(2):609–622. https://doi.org/10.3233/jad-210461
Madsen K, Neumann WJ, Holst K, Marner L, Haahr MT, Lehel S, Knudsen GM, Hasselbalch SG (2011) Cerebral serotonin 4 receptors and amyloid-β in early Alzheimer’s disease. J Alzheimers Dis 26(3):457–466. https://doi.org/10.3233/jad-2011-110056
Mahajan UV, Varma VR, Griswold ME, Blackshear CT, An Y, Oommen AM, Varma S, Troncoso JC, Pletnikova O, O’Brien R, Hohman TJ, Legido-Quigley C, Thambisetty M (2020) Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: a targeted metabolomic and transcriptomic study. PLoS Med 17(1):e1003012. https://doi.org/10.1371/journal.pmed.1003012
Mercado NM, Zhang G, Ying Z, Gomez-Pinilla F (2022) Traumatic brain injury alters the gut-derived serotonergic system and associated peripheral organs. Biochim Biophys Acta Mol Basis Dis 1868(11):166491. https://doi.org/10.1016/j.bbadis.2022.166491
Merighi S, Borea PA, Varani K, Vincenzi F, Jacobson KA, Gessi S (2022) A(2A) Adenosine receptor antagonists in neurodegenerative diseases. Curr Med Chem 29(24):4138–4151. https://doi.org/10.2174/0929867328666211129122550
Metaxas A, Anzalone M, Vaitheeswaran R, Petersen S, Landau AM, Finsen B (2019) Neuroinflammation and amyloid-beta 40 are associated with reduced serotonin transporter (SERT) activity in a transgenic model of familial Alzheimer's disease. Alzheimers Res Ther 11(1):38. https://doi.org/10.1186/s13195-019-0491-2
Mielke MM, Hagen CE, Wennberg AMV, Airey DC, Savica R, Knopman DS, Machulda MM, Roberts RO, Jack CR Jr, Petersen RC, Dage JL (2017) Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the Mayo Clinic Study on aging. JAMA Neurol 74(9):1073–1080. https://doi.org/10.1001/jamaneurol.2017.1359
Mihara A, Ohara T, Hata J, Chen S, Honda T, Tamrakar S, Isa A, Wang D, Shimizu K, Katakura Y, Yonemoto K, Nakao T, Kitazono T, Ninomiya T (2022) Association of serum s-adenosylmethionine, s-adenosylhomocysteine, and their ratio with the risk of dementia and death in a community. Sci Rep 12(1):12427. https://doi.org/10.1038/s41598-022-16242-y
Muguruma Y, Tsutsui H, Akatsu H, Inoue K (2020) Comprehensive quantification of purine and pyrimidine metabolism in Alzheimer’s disease postmortem cerebrospinal fluid by LC-MS/MS with metal-free column. Biomed Chromatogr 34(2):e4722. https://doi.org/10.1002/bmc.4722
Mullan K, Cardwell CR, McGuinness B, Woodside JV, McKay GJ (2018) Plasma antioxidant status in patients with Alzheimer’s disease and cognitively intact elderly: a meta-analysis of case-control studies. J Alzheimers Dis 62(1):305–317. https://doi.org/10.3233/jad-170758
Munasinghe M, Afshari R, Heydarian D, Almotayri A, Dias DA, Thomas J, Jois M (2022) Effects of cocoa on altered metabolite levels in purine metabolism pathways and urea cycle in Alzheimer’s disease in C. elegans. Translational Medicine of Aging 6:14–24. https://doi.org/10.1016/j.tma.2022.10.001
Nho K, Kueider-Paisley A, MahmoudianDehkordi S, Arnold M, Risacher SL, Louie G, Blach C, Baillie R, Han X, Kastenmüller G, Jia W, Xie G, Ahmad S, Hankemeier T, van Duijn CM, Trojanowski JQ, Shaw LM, Weiner MW, Doraiswamy PM, Saykin AJ, Kaddurah-Daouk R (2019) Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: relationship to neuroimaging and CSF biomarkers. Alzheimers Dement 15(2):232–244. https://doi.org/10.1016/j.jalz.2018.08.012
Nho K, Kueider-Paisley A, Arnold M, MahmoudianDehkordi S, Risacher SL, Louie G, Blach C, Baillie R, Han X, Kastenmüller G, Doraiswamy PM, Kaddurah-Daouk R, Saykin AJ (2021) Serum metabolites associated with brain amyloid beta deposition, cognition and dementia progression. Brain Commun 3(3):fcab139. https://doi.org/10.1093/braincomms/fcab139
Niedowicz DM, Reeves VL, Platt TL, Kohler K, Beckett TL, Powell DK, Lee TL, Sexton TR, Song ES, Brewer LD, Latimer CS, Kraner SD, Larson KL, Ozcan S, Norris CM, Hersh LB, Porter NM, Wilcock DM, Murphy MP (2014) Obesity and diabetes cause cognitive dysfunction in the absence of accelerated β-amyloid deposition in a novel murine model of mixed or vascular dementia. Acta Neuropathol Commun 2:64. https://doi.org/10.1186/2051-5960-2-64
Obeid R, Herrmann W (2006) Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett 580(13):2994–3005. https://doi.org/10.1016/j.febslet.2006.04.088
Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM (2003a) Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging 24(8):1063–1070. https://doi.org/10.1016/j.neurobiolaging.2003.08.012
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003b) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39(3):409–421. https://doi.org/10.1016/s0896-6273(03)00434-3
Oxenkrug G (2005) Antioxidant effects of N-acetylserotonin: possible mechanisms and clinical implications. Ann N Y Acad Sci 1053:334–347. https://doi.org/10.1196/annals.1344.029
Pang K, Jiang R, Zhang W, Yang Z, Li LL, Shimozawa M, Tambaro S, Mayer J, Zhang B, Li M, Wang J, Liu H, Yang A, Chen X, Liu J, Winblad B, Han H, Jiang T, Wang W, Nilsson P, Guo W, Lu B (2022) An App knock-in rat model for Alzheimer’s disease exhibiting Aβ and tau pathologies, neuronal death and cognitive impairments. Cell Res 32(2):157–175. https://doi.org/10.1038/s41422-021-00582-x
Park JH, Hong JH, Lee SW, Ji HD, Jung JA, Yoon KW, Lee JI, Won KS, Song BI, Kim HW (2019) The effect of chronic cerebral hypoperfusion on the pathology of Alzheimer’s disease: a positron emission tomography study in rats. Sci Rep 9(1):14102. https://doi.org/10.1038/s41598-019-50681-4
Parodi-Rullán RM, Javadov S, Fossati S (2021) Dissecting the crosstalk between endothelial mitochondrial damage, vascular inflammation, and neurodegeneration in cerebral amyloid angiopathy and Alzheimer’s disease. Cells 10(11):2903. https://doi.org/10.3390/cells10112903
Peña-Bautista C, Flor L, López-Nogueroles M, García L, Ferrer I, Baquero M, Vento M, Cháfer-Pericás C (2020) Plasma alterations in cholinergic and serotonergic systems in early Alzheimer disease: diagnosis utility. Clin Chim Acta 500:233–240. https://doi.org/10.1016/j.cca.2019.10.023
Pietroboni AM, Colombi A, Carandini T, Scarpini E, Galimberti D, Bozzali M (2020) The role of Amyloid-β in white matter damage: possible common pathogenetic mechanisms in neurodegenerative and demyelinating diseases. J Alzheimers Dis 78(1):13–22. https://doi.org/10.3233/jad-200868
Ribeiro JA (1995) Purinergic inhibition of neurotransmitter release in the central nervous system. Pharmacol Toxicol 77(5):299–305. https://doi.org/10.1111/j.1600-0773.1995.tb01031.x
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43(7):e47. https://doi.org/10.1093/nar/gkv007
Rudnitskaya EA, Maksimova KY, Muraleva NA, Logvinov SV, Yanshole LV, Kolosova NG, Stefanova NA (2015) Beneficial effects of melatonin in a rat model of sporadic Alzheimer’s disease. Biogerontology 16(3):303–316. https://doi.org/10.1007/s10522-014-9547-7
Rule RR, Suhy J, Schuff N, Gelinas DF, Miller RG, Weiner MW (2004) Reduced NAA in motor and non-motor brain regions in amyotrophic lateral sclerosis: a cross-sectional and longitudinal study. Amyotroph Lateral Scler Other Motor Neuron Disord 5(3):141–149. https://doi.org/10.1080/14660820410017109
Rutsch A, Kantsjö JB, Ronchi F (2020) The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol 11:604179. https://doi.org/10.3389/fimmu.2020.604179
Sadia K, Leonel Javeres MN, Tahir F, Shah ST, Habib R, Muneer Z, Hussain S, Nurulain SM (2023) Acetylcholinesterase pro-inflammatory cytokines and association of ACHE SNP rs 17228602 with male infertility. PLOS ONE 18(4):e0282579. https://doi.org/10.1371/journal.pone.0282579
Sanchez-Melgar A, Albasanz JL, Grinan-Ferre C, Pallas M, Martin M (2020) Adenosine and metabotropic glutamate receptors are present in blood serum and exosomes from SAMP8 mice: modulation by aging and resveratrol. Cells 9(7):1628. https://doi.org/10.3390/cells9071628
Sánchez-Melgar A, Albasanz JL, Pallàs M, Martín M (2020) Adenosine metabolism in the cerebral cortex from several mice models during aging. Int J Mol Sci 21(19):7300. https://doi.org/10.3390/ijms21197300
Shea TB, Chan A (2008) S-adenosyl methionine: a natural therapeutic agent effective against multiple hallmarks and risk factors associated with Alzheimer’s disease. J Alzheimers Dis 13(1):67–70. https://doi.org/10.3233/jad-2008-13107
Shen L, Chen Y, Yang A, Chen C, Liao L, Li S, Ying M, Tian J, Liu Q, Ni J (2016) Redox proteomic profiling of specifically carbonylated proteins in the serum of triple transgenic Alzheimer’s disease mice. Int J Mol Sci 17(4):469. https://doi.org/10.3390/ijms17040469
Shen L, Xia S, Zhang H, Yao F, Liu X, Zhao Y, Ying M, Liu Q (2019) Precision medicine: role of biomarkers in early prediction and diagnosis of Alzheimers disease. IntechOpen, London
Shen L, Tang X, Zhang H, Zhuang H, Lin J, Zhao Y, Liu X (2023) Targeted metabolomic analysis of the eye tissue of triple transgenic Alzheimer’s disease mice at an early pathological stage. Mol Neurobiol. https://doi.org/10.1007/s12035-023-03533-2
Shin JY, Choi EY, Kim M, Lee HK, Byeon SH (2021) Changes in retinal microvasculature and retinal layer thickness in association with apolipoprotein E genotype in Alzheimer’s disease. Sci Rep 11(1):1847. https://doi.org/10.1038/s41598-020-80892-z
Silva AC, Lemos C, Gonçalves FQ, Pliássova AV, Machado NJ, Silva HB, Canas PM, Cunha RA, Lopes JP, Agostinho P (2018) Blockade of adenosine A(2A) receptors recovers early deficits of memory and plasticity in the triple transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 117:72–81. https://doi.org/10.1016/j.nbd.2018.05.024
Silvestro S, Bramanti P, Mazzon E (2019) Role of miRNAs in Alzheimer’s disease and possible fields of application. Int J Mol Sci 20(16):3979. https://doi.org/10.3390/ijms20163979
Simard T, Jung R, Labinaz A, Faraz MA, Ramirez FD, Di Santo P, Perry-Nguyen D, Pitcher I, Motazedian P, Gaudet C, Rochman R, Marbach J, Boland P, Sarathy K, Alghofaili S, Russo JJ, Couture E, Promislow S, Beanlands RS, Hibbert B (2019) Evaluation of plasma adenosine as a marker of cardiovascular risk: analytical and biological considerations. J Am Heart Assoc 8(15):e012228. https://doi.org/10.1161/jaha.119.012228
Smith GS, Barrett FS, Joo JH, Nassery N, Savonenko A, Sodums DJ, Marano CM, Munro CA, Brandt J, Kraut MA, Zhou Y, Wong DF, Workman CI (2017) Molecular imaging of serotonin degeneration in mild cognitive impairment. Neurobiol Dis 105:33–41. https://doi.org/10.1016/j.nbd.2017.05.007
Snyder SH (1985) Adenosine as a neuromodulator. Annu Rev Neurosci 8:103–124. https://doi.org/10.1146/annurev.ne.08.030185.000535
Snyder HM, Carrillo MC, Grodstein F, Henriksen K, Jeromin A, Lovestone S, Mielke MM, O’Bryant S, Sarasa M, Sjogren M, Soares H, Teeling J, Trushina E, Ward M, West T, Bain LJ, Shineman DW, Weiner M, Fillit HM (2014) Developing novel blood-based biomarkers for Alzheimer’s disease. Alzheimers Dement 10(1):109–114. https://doi.org/10.1016/j.jalz.2013.10.007
Sun C, Gao M, Wang F, Yun Y, Sun Q, Guo R, Yan C, Sun X, Li Y (2020) Serum metabolomic profiling in patients with Alzheimer disease and amnestic mild cognitive impairment by GC/MS. Biomed Chromatogr 34(9):e4875. https://doi.org/10.1002/bmc.4875
Sun YY, Wang Z, Zhou HY, Huang HC (2022) Sleep-wake disorders in Alzheimer’s disease: a review. ACS Chem Neurosci 13(10):1467–1478. https://doi.org/10.1021/acschemneuro.2c00097
Sundman MH, Chen NK, Subbian V, Chou YH (2017) The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun 66:31–44. https://doi.org/10.1016/j.bbi.2017.05.009
Tan PK, Farrar JE, Gaucher EA, Miner JN (2016) Coevolution of URAT1 and uricase during primate evolution: implications for serum urate homeostasis and gout. Mol Biol Evol 33(9):2193–2200. https://doi.org/10.1093/molbev/msw116
Teruya T, Chen YJ, Kondoh H, Fukuji Y, Yanagida M (2021) Whole-blood metabolomics of dementia patients reveal classes of disease-linked metabolites. Proc Natl Acad Sci U S A 118(37):e2022857118. https://doi.org/10.1073/pnas.2022857118
Tohgi H, Abe T, Takahashi S, Kimura M, Takahashi J, Kikuchi T (1992) Concentrations of serotonin and its related substances in the cerebrospinal fluid in patients with Alzheimer type dementia. Neurosci Lett 141(1):9–12. https://doi.org/10.1016/0304-3940(92)90322-x
Toledo JB, Arnold M, Kastenmüller G, Chang R, Baillie RA, Han X, Thambisetty M, Tenenbaum JD, Suhre K, Thompson JW, John-Williams LS, MahmoudianDehkordi S, Rotroff DM, Jack JR, Motsinger-Reif A, Risacher SL, Blach C, Lucas JE, Massaro T, Louie G, Zhu H, Dallmann G, Klavins K, Koal T, Kim S, Nho K, Shen L, Casanova R, Varma S, Legido-Quigley C, Moseley MA, Zhu K, Henrion MYR, van der Lee SJ, Harms AC, Demirkan A, Hankemeier T, van Duijn CM, Trojanowski JQ, Shaw LM, Saykin AJ, Weiner MW, Doraiswamy PM, Kaddurah-Daouk R (2017) Metabolic network failures in Alzheimer’s disease: a biochemical road map. Alzheimers Dement 13(9):965–984. https://doi.org/10.1016/j.jalz.2017.01.020
Uddin MS, Tewari D, Mamun AA, Kabir MT, Niaz K, Wahed MII, Barreto GE, Ashraf GM (2020) Circadian and sleep dysfunction in Alzheimer’s disease. Ageing Res Rev 60:101046. https://doi.org/10.1016/j.arr.2020.101046
van Calker D, Biber K, Domschke K, Serchov T (2019) The role of adenosine receptors in mood and anxiety disorders. J Neurochem 151(1):11–27. https://doi.org/10.1111/jnc.14841
van der Lee SJ, Teunissen CE, Pool R, Shipley MJ, Teumer A, Chouraki V, Melo van Lent D, Tynkkynen J, Fischer K, Hernesniemi J, Haller T, Singh-Manoux A, Verhoeven A, Willemsen G, de Leeuw FA, Wagner H, van Dongen J, Hertel J, Budde K, Willems van Dijk K, Weinhold L, Ikram MA, Pietzner M, Perola M, Wagner M, Friedrich N, Slagboom PE, Scheltens P, Yang Q, Gertzen RE, Egert S, Li S, Hankemeier T, van Beijsterveldt CEM, Vasan RS, Maier W, Peeters CFW, Jörgen Grabe H, Ramirez A, Seshadri S, Metspalu A, Kivimäki M, Salomaa V, Demirkan A, Boomsma DI, van der Flier WM, Amin N, van Duijn CM (2018) Circulating metabolites and general cognitive ability and dementia: evidence from 11 cohort studies. Alzheimers Dement 14(6):707–722. https://doi.org/10.1016/j.jalz.2017.11.012
Varma VR, Oommen AM, Varma S, Casanova R, An Y, Andrews RM, O’Brien R, Pletnikova O, Troncoso JC, Toledo J, Baillie R, Arnold M, Kastenmueller G, Nho K, Doraiswamy PM, Saykin AJ, Kaddurah-Daouk R, Legido-Quigley C, Thambisetty M (2018) Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: a targeted metabolomics study. PLoS Med 15(1):e1002482. https://doi.org/10.1371/journal.pmed.1002482
Vasudha KC, Kumar AN, Venkatesh T (2006) Studies on the age dependent changes in serum adenosine deaminase activity and its changes in hepatitis. Indian J Clin Biochem 21(1):116–120. https://doi.org/10.1007/bf02913078
Venkataraman AV, Mansur A, Rizzo G, Bishop C, Lewis Y, Kocagoncu E, Lingford-Hughes A, Huiban M, Passchier J, Rowe JB, Tsukada H, Brooks DJ, Martarello L, Comley RA, Chen L, Schwarz AJ, Hargreaves R, Gunn RN, Rabiner EA, Matthews PM (2022) Widespread cell stress and mitochondrial dysfunction occur in patients with early Alzheimer’s disease. Sci Transl Med 14(658):eabk1051. https://doi.org/10.1126/scitranslmed.abk1051
Vogt NM, Romano KA, Darst BF, Engelman CD, Johnson SC, Carlsson CM, Asthana S, Blennow K, Zetterberg H, Bendlin BB, Rey FE (2018) The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res Ther 10(1):124. https://doi.org/10.1186/s13195-018-0451-2
Wang G, Zhou Y, Huang FJ, Tang HD, Xu XH, Liu JJ, Wang Y, Deng YL, Ren RJ, Xu W, Ma JF, Zhang YN, Zhao AH, Chen SD, Jia W (2014) Plasma metabolite profiles of Alzheimer’s disease and mild cognitive impairment. J Proteome Res 13(5):2649–2658. https://doi.org/10.1021/pr5000895
Whiley L, Chappell KE, D’Hondt E, Lewis MR, Jiménez B, Snowden SG, Soininen H, Kłoszewska I, Mecocci P, Tsolaki M, Vellas B, Swann JR, Hye A, Lovestone S, Legido-Quigley C, Holmes E (2021) Metabolic phenotyping reveals a reduction in the bioavailability of serotonin and kynurenine pathway metabolites in both the urine and serum of individuals living with Alzheimer’s disease. Alzheimers Res Ther 13(1):20. https://doi.org/10.1186/s13195-020-00741-z
Willette AA, Pappas C, Hoth N, Wang Q, Klinedinst B, Willette SA, Larsen B, Pollpeter A, Li T, Le S, Collazo-Martinez AD, Mochel JP, Allenspach K, Dantzer R (2021) Alzheimer's disease neuroimaging initiative. inflammation, negative affect, and amyloid burden in alzheimer's disease: insights from the kynurenine pathway. Brain Behav Immun 95:216–225. https://doi.org/10.1016/j.bbi.2021.03.019
Woods LT, Ajit D, Camden JM, Erb L, Weisman GA (2016) Purinergic receptors as potential therapeutic targets in Alzheimer’s disease. Neuropharmacology 104:169–179. https://doi.org/10.1016/j.neuropharm.2015.10.031
Xie K, Qin Q, Long Z, Yang Y, Peng C, Xi C, Li L, Wu Z, Daria V, Zhao Y, Wang F, Wang M (2021) High-throughput metabolomics for discovering potential biomarkers and identifying metabolic mechanisms in aging and Alzheimer’s disease. Front Cell Dev Biol 9:602887. https://doi.org/10.3389/fcell.2021.602887
Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161(2):264–276. https://doi.org/10.1016/j.cell.2015.02.047
Yin C, Harms AC, Hankemeier T, Kindt A, de Lange ECM (2023) Status of metabolomic measurement for insights in Alzheimer’s disease progression—What is missing? Int J Mol Sci 24(5):4960. https://doi.org/10.3390/ijms24054960
Yu JT, Chang RC, Tan L (2009) Calcium dysregulation in Alzheimer’s disease: from mechanisms to therapeutic opportunities. Prog Neurobiol 89(3):240–255. https://doi.org/10.1016/j.pneurobio.2009.07.009
Yu W, Yu W, Yang Y, Lü Y (2021) Exploring the key genes and identification of potential diagnosis biomarkers in Alzheimer’s disease using bioinformatics analysis. Front Aging Neurosci 13:602781. https://doi.org/10.3389/fnagi.2021.602781
Zhang H, Zhao Y, Zhao D, Chen X, Khan NU, Liu X, Zheng Q, Liang Y, Zhu Y, Iqbal J, Lin J, Shen L (2021a) Potential biomarkers identified in plasma of patients with gestational diabetes mellitus. Metabolomics 17(11):99. https://doi.org/10.1007/s11306-021-01851-x
Zhang L, Li L, Meng F, Yu J, He F, Lin Y, Su Y, Hu M, Liu X, Liu Y, Luo B, Peng G (2021b) Serum metabolites differentiate amnestic mild cognitive impairment from healthy controls and predict early Alzheimer’s disease via untargeted lipidomics analysis. Front Neurol 12:704582. https://doi.org/10.3389/fneur.2021.704582
Zhao Y, Qian R, Zhang J, Liu F, Iqbal K, Dai CL, Gong CX (2020) Young blood plasma reduces Alzheimer’s disease-like brain pathologies and ameliorates cognitive impairment in 3xTg-AD mice. Alzheimers Res Ther 12(1):70. https://doi.org/10.1186/s13195-020-00639-w
Zhao L, Teng J, Mai W, Su J, Yu B, Nong X, Li C, Wei Y, Duan G, Deng X, Deng D, Chen S (2021a) A pilot study on the cutoff value of related brain metabolite in Chinese elderly patients with mild cognitive impairment using MRS. Front Aging Neurosci 13:617611. https://doi.org/10.3389/fnagi.2021.617611
Zhao Y, Chen H, Iqbal J, Liu X, Zhang H, Xiao S, Jin N, Yao F, Shen L (2021b) Targeted metabolomics study of early pathological features in hippocampus of triple transgenic Alzheimer’s disease male mice. J Neurosci Res 99(3):927–946. https://doi.org/10.1002/jnr.24750
Zhou J, Yin Y (2016) Strategies for large-scale targeted metabolomics quantification by liquid chromatography-mass spectrometry. Analyst 141(23):6362–6373. https://doi.org/10.1039/c6an01753c
Zong B, Yu F, Zhang X, Zhao W, Sun P, Li S, Li L (2022) Understanding how physical exercise improves Alzheimer’s disease: cholinergic and monoaminergic systems. Front Aging Neurosci 14:869507. https://doi.org/10.3389/fnagi.2022.869507
Fioravanti M, Yanagi M (2000) Cytidinediphosphocholine (CDP choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst Rev (2):Cd000269. https://doi.org/10.1002/14651858.Cd000269
Fioravanti M, Yanagi M (2004) Cytidinediphosphocholine (CDP choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst Rev (2):Cd000269. https://doi.org/10.1002/14651858.CD000269.pub2
Hornedo-Ortega R, Da Costa G, Cerezo AB, Troncoso AM, Richard T, Garcia-Parrilla MC (2018) In vitro effects of serotonin, melatonin, and other related indole compounds on amyloid-β kinetics and neuroprotection. Mol Nutr Food Res 62(3). https://doi.org/10.1002/mnfr.201700383
Kumar V, Giacobini E, Markwell S (1989) CSF choline and acetylcholinesterase in early-onset vs. late-onset Alzheimer’s disease patients. Acta Neurol Scand 80(5):461–466. https://doi.org/10.1111/j.1600-0404.1989.tb03910.x
Lin B, Ma H, Zhang K, Cui J (2022) Regulatory mechanisms and metabolic changes of miRNA during leaf color change in the bud mutation branches of Acer pictum subsp. mono. Front Plant Sci 13:1047452. https://doi.org/10.3389/fpls.2022.1047452
Oxenkrug G, van der Hart M, Roeser J, Summergrad P (2017) Peripheral Tryptophan—Kynurenine metabolism associated with metabolic syndrome is different in Parkinson’s and Alzheimer’s diseases. Endocrinol Diabetes Metab J 1(4)
Southern ŠO, Stallings JD, Ippolito DL (2015) A systems biology approach to heat stress, heat injury, and heat stroke. In: Paper presented at the Advances in Global Health through Sensing Technologies 2015
Acknowledgements
This study was supported by the National Key Research and Development Program of China (No. 2018YFE0118900), the National Natural Science Foundation of China (Grant No. 31870825), the Shenzhen Bureau of Science, Technology and Information (No. JCYJ20170412110026229) and the Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions (2023SHIBS0003). We thank the Center for Instrumental Analysis, Shenzhen University.
Author information
Authors and Affiliations
Contributions
H.Z. wrote the manuscript and analyzed the most of data. X.C. contributed to revision; X.T, Y.Z., H.Y., and Z.L. prepared material, reagents, and instrumentation. X.Y., X.C., and X.F. interpreted the results. L.S. conceived of the study and designed the study. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling editor: E. Closs.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhuang, H., Cao, X., Tang, X. et al. Investigating metabolic dysregulation in serum of triple transgenic Alzheimer’s disease male mice: implications for pathogenesis and potential biomarkers. Amino Acids 56, 10 (2024). https://doi.org/10.1007/s00726-023-03375-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00726-023-03375-1