Abstract
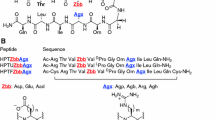
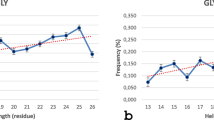
The charge-containing hydrophilic functionalities of encoded charged amino acids are linked to the backbone via different numbers of hydrophobic methylenes, despite the apparent electrostatic nature of protein ion pairing interactions. To investigate the effect of side chain length of guanidinium- and carboxylate-containing residues on ion pairing interactions, α-helical peptides containing Zbb–Xaa (i, i + 3), (i, i + 4) and (i, i + 5) (Zbb = carboxylate-containing residues Aad, Glu, Asp in decreasing length; Xaa = guanidinium residues Agh, Arg, Agb, Agp in decreasing length) sequence patterns were studied by circular dichroism spectroscopy (CD). The helicity of Aad- and Glu-containing peptides was similar and mostly pH independent, whereas the helicity of Asp-containing peptides was mostly pH dependent. Furthermore, the Arg-containing peptides consistently exhibited higher helicity compared to the corresponding Agp-, Agb-, and Agh-containing peptides. Side chain conformational analysis by molecular mechanics calculations showed that the Zbb–Xaa (i, i + 3) and (i, i + 4) interactions mainly involved the χ 1 dihedral combinations (g+, g+) and (g−, g+), respectively. These low energy conformations were also observed in intrahelical Asp–Arg and Glu–Arg salt bridges of natural proteins. Accordingly, Asp and Glu provides variation in helix characteristics associated with Arg, but Aad does not provide features beyond those already delivered by Glu. Importantly, nature may have chosen the side chain length of Arg to support helical conformations through inherent high helix propensity coupled with stabilizing intrahelical ion pairing interactions with the carboxylate-containing residues.






Similar content being viewed by others
Abbreviations
- Aad:
-
(S)-aminoadipate
- Agb:
-
(S)-2-amino-4-guanidinobutyric acid
- Agh:
-
(S)-2-amino-6-guanidinohexanoic acid
- Agp:
-
(S)-2-amino-3-guanidinopropionic acid
- Ala:
-
Alanine
- Arg:
-
Arginine
- Asp:
-
Aspartate
- CD:
-
Circular dichroism spectroscopy
- Fmoc:
-
N α-fluorenylmethyloxycarbonyl
- Glu:
-
Glutamate
- Lys:
-
Lysine
- MALDI-TOF:
-
Matrix-assisted laser desorption ionization time-of-flight
- Tyr:
-
Tyrosine
References
Altman M, Lee P, Rich A, Zhang S (2000) Conformational behavior of ionic self-complementary peptides. Protein Sci 9(6):1095–1105
Andrew CD, Penel S, Jones GR, Doig AJ (2001) Stabilizing nonpolar/polar side-chain interactions in the α-helix. Proteins Struct Funct Genet 45(4):449–455
Anfinsen CB (1973) Principles that govern folding of protein chains. Science 181(4096):223–230. doi:10.1126/Science.181.4096.223
Atherton E, Fox H, Harkiss D, Logan CJ, Sheppard RC, Williams BJ (1978) Mild procedure for solid phase peptide synthesis—use of fluorenylmethoxycarbonylamino-acids. J Chem Soc Chem Commun 13:537–539. doi:10.1039/C39780000537
Baldwin RL, Rose GD (1999a) Is protein folding hierarchic? I. Local structure and peptide folding. Trends Biochem Sci 24(1):26–33. doi:10.1016/S0968-0004(98)01346-2
Baldwin RL, Rose GD (1999b) Is protein folding hierarchic? II. Folding intermediates and transition states. Trends Biochem Sci 24(2):77–83. doi:10.1016/S0968-0004(98)01345-0
Barlow DJ, Thornton JM (1983) Ion-pairs in proteins. J Mol Biol 168(4):867–885
Chakrabartty A, Kortemme T, Padmanabhan S, Baldwin RL (1993) Aromatic side-chain contribution to far-ultraviolet circular-dichroism of helical peptides and its effect on measurement of helix propensities. Biochemistry 32(21):5560–5565. doi:10.1021/Bi00072a010
Chakrabartty A, Kortemme T, Baldwin RL (1994) Helix propensities of the amino acids measured in alanine-based peptides without helix-stabilizing side-chain interactions. Protein Sci 3(5):843–852. doi:10.1002/pro.5560030514
Chang CT, Wu C-SC, Yang JT (1978) Circular dichroic analysis of protein conformation: inclusion of the β-turns. Anal Biochem 91(1):13–31. doi:10.1016/0003-2697(78)90812-6
Cheng RP, Girinath P, Ahmad R (2007) Effect of lysine side chain length on intra-helical glutamate-lysine ion pairing interactions. Biochemistry 46:10528–10537
Cheng RP, Girinath P, Suzuki Y, Kuo H-T, Hsu H-C, Wang W-R, Yang P-A, Gullickson D, Wu C-H, Koyack MJ, Chiu H-P, Weng Y-J, Hart P, Kokona B, Fairman R, Lin T-E, Barrett O (2010) Positional effects on helical Ala-based peptides. Biochemistry 49:9372–9384
Cheng RP, Wang W-R, Girinath P, Yang P-A, Ahmad R, Li J-H, Hart P, Kokona B, Fairman R, Kilpatrick C, Argiros A (2012a) Effect of glutamate side chain length on intrahelical glutamate-lysine ion pairing interactions. Biochemistry 51(36):7157–7172. doi:10.1021/bi300655z
Cheng RP, Weng Y-J, Wang W-R, Koyack MJ, Suzuki Y, Wu C-H, Yang PA, Hsu H-C, Kuo H-T, Girinath P, Fang C-J (2012b) Helix formation and capping energetics of arginine analogs with varying side chain length. Amino Acids 43(1):195–206. doi:10.1007/s00726-011-1064-2
Creighton TE (1993) Proteins, structure and molecular properties, 2nd edn. W. H. Freeman and Co., New York
Dill KA (1990) Dominant forces in protein folding. Biochemistry 29(31):7133–7155
Dill KA, Ozkan SB, Shell MS, Weikl TR (2008) The protein folding problem. Annu Rev Biophys 37:289–316. doi:10.1146/Annurev.Biophys.37.092707.153558
Doig AJ, Baldwin RL (1995) N- and C-capping preferences for all 20 amino acids in α-helical peptides. Protein Sci 4(7):1325–1336. doi:10.1002/pro.5560040708
Dominy BN, Minoux H, Brooks CL (2004) An electrostatic basis for the stability of thermophilic proteins. Proteins 57(1):128–141. doi:10.1002/Prot.20190
Donald JE, Kulp DW, DeGrado WF (2011) Salt bridges: geometrically specific, designable interactions. Proteins 79(3):898–915. doi:10.1002/Prot.22927
Dunbrack RL, Karplus M (1993) Backbone-dependent rotamer library for proteins—application to side-chain prediction. J Mol Biol 230(2):543–574
Edelhoch H (1967) Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6(7):1948–1954
Elcock AH (1998) The stability of salt bridges at high temperatures: implications for hyperthermophilic proteins. J Mol Biol 284(2):489–502. doi:10.1006/Jmbi.1998.2159
Engel DE, DeGrado WF (2004) Amino acid propensities are position-dependent throughout the length of α-helices. J Mol Biol 337:1195–1205
Fersht AR (1999) Structure and mechanism in protein science. A guide to enzyme catalysis and protein folding. W.H. Freeman and Co., New York
Fields GB, Noble RL (1990) Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res 35(3):161–214
Garcia AE, Sanbonmatsu KY (2002) α-Helical stabilization by side chain shielding of backbone hydrogen bonds. Proc Natl Acad Sci USA 99(5):2782–2787. doi:10.1073/Pnas.042496899
Griep S, Hobohm U (2010) PDBselect 1992–2009 and PDBfilter-select. Nucleic Acids Res 38:D318–D319
Groebke K, Renold P, Tsang KY, Allen TJ, McClure KF, Kemp DS (1996) Template-nucleated alanine-lysine helices are stabilized by position-dependent interactions between the lysine side chain and the helix barrel. Proc Natl Acad Sci USA 93(9):4025–4029. doi:10.1073/Pnas.93.9.4025
Gunasekaran K, Nagarajaram HA, Ramakrishnan C, Balaram P (1998) Stereochemical punctuation marks in protein structures: glycine and proline containing helix stop signals. J Mol Biol 275(5):917–932
Haas DH, Murphy RM (2004a) Design of a pH-sensitive pore-forming peptide with improved performance. J Pept Res 63(1):9–16
Haas DH, Murphy RM (2004b) Templated assembly of the pH-sensitive membrane-lytic peptide GALA. J Pept Res 63(6):451–459
Hirosue S, Weber T (2006) pH-dependent lytic peptides discovered by phage display. Biochemistry 45(20):6476–6487
Hobohm U, Sander C (1994) Enlarged representative set of protein structures. Protein Sci 3(3):522–524. doi:10.1002/pro.5560030317
Hong YS, Legge RL, Zhang S, Chen P (2003) Effect of amino acid sequence and pH on nanofiber formation of self-assembling peptides EAK16-II and EAK16-IV. Biomacromolecules 4(5):1433–1442
Huyghues-Despointes BM, Scholtz JM, Baldwin RL (1993a) Effect of a single aspartate on helix stability at different positions in a neutral alanine-based peptide. Protein Sci 2(10):1604–1611
Huyghues-Despointes BM, Scholtz JM, Baldwin RL (1993b) Helical peptides with three pairs of Asp–Arg and Glu–Arg residues in different orientations and spacings. Protein Sci 2(1):80–85. doi:10.1002/pro.5560020108
Jiang YL, Ichikawa Y, Song F, Stivers JT (2003) Powering DNA repair through substrate electrostatic interactions. Biochemistry 42(7):1922–1929. doi:10.1021/bi027014x
Johnsson K, Allemann RK, Widmer H, Benner SA (1993) Synthesis, structure and activity of artificial, rationally designed catalytic polypeptides. Nature 365(6446):530–532
Karshikoff A, Ladenstein R (2001) Ion pairs and the thermotolerance of proteins from hyperthermophiles: a ‘traffic rule’ for hot roads. Trends Biochem Sci 26(9):550–556. doi:10.1016/S0968-0004(01)01918-1
Klingler TM, Brutlag DL (1994) Discovering structural correlations in α-helices. Protein Sci 3(10):1847–1857. doi:10.1002/pro.5560031024
Kobayashi Y, Cardinaux F, Zweifel BO, Scheraga HA (1977) Helix-coil stability constants for the naturally occurring amino acids in water. 16. Aspartic acid parameters from random poly(hydroxybutylglutamine-co-l-aspartic acid). Macromolecules 10:1271–1283
Kuehne J, Murphy RM (2001) Synthesis and characterization of membrane-active GALA-OKT9 conjugates. Bioconjugate Chem 12(5):742–749
Kumar S, Nussinov R (2002) Close-range electrostatic interactions in proteins. ChemBioChem 3(7):604–617. doi:10.1002/1439-7633(20020703)3:7<604:Aid-Cbic604>3.0.Co;2-X
Kuo L-H, Li J-H, Kuo H-T, Hung C-Y, Tsai H-Y, Chiu W-C, Wu C-H, Wang W-R, Yang P-A, Yao Y-C, Wong TW, Huang S-J, Huang S-L, Cheng RP (2013) Effect of charged amino acid side chain length at non-hydrogen bonded strand positions on β-hairpin stability. Biochemistry 52:7785–7797
Lifson S, Roig A (1961) The theory of helix-coil transition in polypeptides. J Chem Phys 34:1963–1974
Lu H, Wang J, Bai Y, Lang JW, Liu S, Lin Y, Cheng J (2011) Ionic polypeptides with unusual helical stability. Nat Commun 2:206
Luo PZ, Baldwin RL (1999) Interaction between water and polar groups of the helix backbone: an important determinant of helix propensities. Proc Natl Acad Sci USA 96(9):4930–4935. doi:10.1073/Pnas.96.9.4930
Makhatadze GI, Privalov PL (1995) Energetics of protein structure. Adv Protein Chem 47:307–425
Malakauskas SM, Mayo SL (1998) Design, structure and stability of a hyperthermophilic protein variant. Nat Struct Biol 5(6):470–475. doi:10.1038/Nsb0698-470
Marqusee S, Baldwin RL (1987) Helix stabilization by Glu-…Lys+ salt bridges in short peptides of de novo design. Proc Natl Acad Sci USA 84(24):8898–8902
Maxfield FR, Scheraga HA (1975) The effect of neighboring charges on the helix forming ability of charged amino acids in proteins. Macromolecules 8(4):491–493
McGregor MJ, Islam SA, Sternberg MJE (1987) Analysis of the relationship between side-chain conformation and secondary structure in globular-proteins. J Mol Biol 198(2):295–310
Pace CN, Scholtz JM (1998) A helix propensity scale based on experimental studies of peptides and proteins. Biophys J 75(1):422–427
Pace CN (2000) Single surface stabilizer. Nat Struct Biol 7(5):345–346. doi:10.1038/75100
Pace CN, Vajdos F, Fee L, Grimsley G, Gray T (1995) How to measure and predict the molar absorption-coefficient of a protein. Protein Sci 4(11):2411–2423
Pace CN, Shirley BA, McNutt M, Gajiwala K (1996) Forces contributing to the conformational stability of proteins. FASEB J 10(1):75–83
Padmanabhan S, York EJ, Stewart JM, Baldwin RL (1996) Helix propensities of basic amino acids increase with the length of the side-chain. J Mol Biol 257(3):726–734. doi:10.1006/jmbi.1996.0197
Pagel K, Koksch B (2008) Following polypeptide folding and assembly with conformational switches. Curr Opin Chem Biol 12:730–739
Pauling L, Corey RB, Branson HR (1951) The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci USA 37(4):205–211
Pepe-Mooney BJ, Fairman R (2009) Peptides as materials. Curr Opin Struct Biol 19:483–494
Robert CH (1990) A hierarchical nesting approach to describe the stability of α-helices with side-chain interactions. Biopolymers 30(3–4):335–347
Robinson-Rechavi M, Alibés A, Godzik A (2006) Contribution of electrostatic interactions, compactness and quaternary structure to protein thermostability: lessons from structural genomics of Thermotoga maritima. J Mol Biol 356(2):547–557. doi:10.1016/J.Jmb.11.065
Rose GD, Fleming PJ, Banavar JR, Maritan A (2006) A backbone-based theory of protein folding. Proc Natl Acad Sci USA 103(45):16623–16633. doi:10.1073/Pnas.0606843103
Sali D, Bycroft M, Fersht AR (1988) Stabilization of protein-structure by interaction of α-helix dipole with a charged side-chain. Nature 335(6192):740–743
Scholtz JM, Qian H, Robbins VH, Baldwin RL (1993) The energetics of ion-pair and hydrogen-bonding interactions in a helical peptide. Biochemistry 32(37):9668–9676
Sheparovych R, Roiter Y, Yang J, Kopecek J, Minko S (2009) Stimuli-responsive properties of peptide-based copolymers studied via directional growth of self-assembled patterns on solid substrate. Biomacromolecules 10:1955–1961
Smith JS, Scholtz JM (1998) Energetics of polar side-chain interactions in helical peptides: salt effects on ion pairs and hydrogen bonds. Biochemistry 37(1):33–40. doi:10.1021/bi972026h
Su JG, Chen WZ, Wang CX (2010) Role of electrostatic interactions for the stability and folding behavior of cold shock protein. Proteins 78(9):2157–2169. doi:10.1002/Prot.22730
Thomas AS, Elcock AH (2004) Molecular simulations suggest protein salt bridges are uniquely suited to life at high temperatures. J Am Chem Soc 126(7):2208–2214. doi:10.1021/Ja039159c
Turk MJ, Reddy JA, Chmielewski JA, Low PS (2002) Characterization of a novel pH-sensitive peptide that enhances drug release from folate-targeted liposomes at endosomal pHs. Biochim Biophys Acta-Biomembr 1559(1):56–68
Xiao L, Honig B (1999) Electrostatic contributions to the stability of hyperthermophilic proteins. J Mol Biol 289(5):1435–1444. doi:10.1006/Jmbi.1999.2810
Yip KS, Britton KL, Stillman TJ, Lebbink J, De Vos WM, Robb FT, Vetriani C, Maeder D, Rice DW (1998) Insights into the molecular basis of thermal stability from the analysis of ion-pair networks in the Glutamate Dehydrogenase family. Eur J Biochem 255(2):336–346. doi:10.1046/J.1432-1327.1998.2550336.X
Zimenkov Y, Dublin SN, Ni R, Tu RS, Breedveld V, Apkarian RP, Conticello VP (2006) Rational design of a reversible pH-responsive switch for peptide self-assembly. J Am Chem Soc 128:6770–6771
Acknowledgments
This work was supported by National Taiwan University (NTU-ERP-103R891302) and the Ministry of Science and Technology in Taiwan (former National Science Council; NSC-97-2113-111-002-019-MY2, NSC-98-2119-M-002-025, NSC-99-2113-M-002-002-MY2, NSC-101-2113-M-002-006-MY2). The authors would like to thank the Computer and Information Networking Center at National Taiwan University for the support of the high-performance computing facilities.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kuo, HT., Yang, PA., Wang, WR. et al. Effect of side chain length on intrahelical interactions between carboxylate- and guanidinium-containing amino acids. Amino Acids 46, 1867–1883 (2014). https://doi.org/10.1007/s00726-014-1737-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1737-8