Abstract
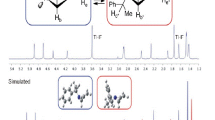
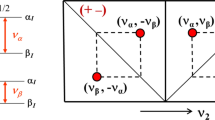
In this work, we carried out an extended verification of the 13C–15N spin–spin coupling constants as a new structural indicator of nitrogen-containing organic compounds. In this regard, we performed a quantum-chemical calculation (B3LYP with basis set 6-311++G(2df,2p)) for a representative sample of 193 spin–spin couplings for the currently known literature experimental data on them in the conformationally rigid and structurally fixed compounds. Comparison of theoretical couplings with experimental ones shows a statistically significant good to excellent agreement. A parallel analysis of the variability of the calculated values of 13C–15N spin bonds with the variability of experimental data within groups of related compounds turned out to be practically useful. The approach developed can be used to quite reasonably determine the signs of spin–spin coupling constants 13C–15N. It can also provide important additional information for assigning 13C peaks in cases where this cannot be done using standard NMR spectroscopy techniques.







Similar content being viewed by others
References
T.D.W. Claridge, High-resolution NMR techniques in organic chemistry, 3rd edn. (Elsevier Science, Oxford, 2016)
D.A. Cheshkov, D.O. Synitsyn, K.F. Sheberstov, V.A. Chertkov, J. Magn. Reson. 272, 10 (2016)
V.A. Chertkov, D.A. Cheshkov, D.O. Sinitsyn, eMagRes 6, 359 (2017)
H. Gunther, N.M.R. Spectroscopy, Basic principles, concepts and applications in chemistry, 3rd edn. (Wiley-VCH, Weinheim, 2013)
R.J. Abraham, M. Mobli, Modelling 1H NMR spectra of organic compounds theory, applications and NMR prediction software (Wiley, Chichester, 2008)
M.B. Kaupp, V.G. Malkin, Calculation of NMR and EPR parameter. Theory and applications (Wiley-VCH, Weinheim, 2004)
J.B. Foresman, A. Frisch, Exploring chemistry with electronic structure methods (Gaussian Inc., Wallingford, 2015)
L.B. Krivdin, R.H. Contreras, Annu. Rep. NMR Spectrosc. 61, 133 (2007)
L.B. Krivdin, Magn. Reson. Chem. 60, 733 (2022)
K. Kamienska-Trela, J. Wójcik, Nucl. Magn. Reson. 42, 181 (2013)
P.E. Hansen, Prog. Nucl. Magn. Reson. 42, 181 (1981)
M.J. Frisch, G.W. Trucks, H.B. Schlegel et al., Gaussian 09W, revision A.02 (Gaussian Inc., Wallingford, 2009)
W. Deng, J.R. Cheeseman, M.J. Frisch, J. Chem. Theory Comput. 2, 1028 (2006)
Y.A. Strelenko, V.N. Torocheshnikov, N.M. Sergeyev, J. Magn. Reson. 89, 123 (1990)
T. Coursindel, D. Farran, J. Martinez, G. Dewynter, Tetrahedron Lett. 49, 906 (2008)
T.S. Shestakova, Z.O. Shenkarev, S.L. Deev, O.N. Chupakhin, I.A. Khalymbadzha, V.L. Rusinov, A.S. Arseniev, J. Org. Chem. 78, 6975 (2013)
T.A. Ganina, D.A. Cheshkov, V.A. Chertkov, Russ. J. Org. Chem. 53, 12 (2017)
T.A. Ganina, V.A. Chertkov, Russ. J. Org. Chem. 52, 489 (2016)
L. Paolillo, E.D. Becker, J. Magn. Reson. 3, 200 (1970)
S. Berger, J.D. Roberts, J. Am. Chem. Soc. 96, 6757 (1974)
S. Berger, S. Braun, 200 and more NMR experiments: a practical course (Wiley-VCH, Oxford, Weinheim, 2004)
V.V. Nalimov, The Theory of the Experiment (Nauka, Moscow, 1977)
P. Diehl, J. Jokisaari, J. Amrein, T. Väänänen, P. Pyykkö, J. Magn. Reson. 48, 495 (1982)
O.W. Sørensen, H. Bildsøe, H.J. Jakobsen, J. Magn. Reson. 45, 325 (1981)
J.S. Figueroa, N.A. Piro, C.R. Clough, C.C. Cummins, J. Am. Chem. Soc. 128, 940 (2006)
W. McFarlane, Mol. Phys. 10, 603 (1966)
J. Levic, R. Micura, Beilstein J. Org. Chem. 10, 1914 (2014)
R.J. Halter, R.L. Fimmen, R.J. McMahon, S.A. Peebles, R.L. Kuczkowski, J.F. Stanton, J. Am. Chem. Soc. 123, 12353 (2001)
A. Laxer, B. Fischer, J. Label. Compd. Radiopharm. 43, 47 (2000)
P.L. Johnson, N.R. Pearson, B. Schuster, J. Cobb, J. Label. Compd. Radiopharm. 52, 382 (2009)
S.J. Weiner, S.M. Holl, D.F. Covey, Magn. Reson. Chem. 32, 122 (1994)
M.M. Guru, T. Shima, Z. Hou, Angew. Chem. 55, 12316 (2016)
S.L. Deev, I.A. Khalymbadzha, T.S. Shestakova, V.N. Charushin, O.N. Chupakhin, RSC Adv. 9, 26856 (2019)
G. Zhang, X. Ren, J. Chen, M. Hu, J. Cheng. Org. Lett. 13, 5004 (2011)
X. Creary, A.F. Sky, G. Phillips, J. Org. Chem. 55, 2005 (1990)
R.D. Wigglesworth, W.T. Raynes, S. Kirpekar, J. Oddershede, S.P.A. Sauer, J. Chem. Phys. 112, 3735 (2000)
T. Khin, G.A. Webb, Org. Magn. Reson. 11, 487 (1978)
R.W. Stephany, M.J.A. De Bie, W. Drenth, Org. Magn. Reson. 6, 45 (1974)
W. McFarlane, J. Chem. Soc. 1660 (1967)
N.J. Koole, D. Knol, M.J.A. De Bie, J. Magn. Reson. 21, 499 (1976)
I. Morishima, A. Mizumo, T. Yonezawa, K. Goto, J. Chem. Soc. Chem. Commun. vol. 1321 (1970)
J.M. Garcia de la Vega, J. San Fabián, in: High resolution NMR Spectroscopy, vol. 3. Elsevier B.V., New York (2013)
T. Axenrod, C.M. Watnick, M.J. Wieder, S. Duangthai, G.A. Webb, H.J.C. Yeh, S. Bulusu, M.M. King, Org. Magn. Reson. 20, 11 (1982)
L. Ernst, E. Lustig, V. Wray, J. Magn. Reson. 22, 459 (1976)
M. Christl, Org. Magn. Reson. 7, 349 (1975)
R.T. Lichter, J.D. Roberts, J. Am. Chem. Soc. 93, 5218 (1971)
T. Wamsler, J.T. Nielsen, E.J. Pedersen, K. Schaumburg, J. Magn. Reson. 31, 177 (1978)
M. Fruchier, V. Pellegrin, R. Schimpf, J. Elguero. Org. Magn. Reson. 18, 10 (1982)
A.K. Shestakova, V.V. Stanishevskiy, V.A. Chertkov, Chem. Heterocycl. Compd. (in press)
T. Axenrod, M.J. Wieder, T. Khin, G.A. Webb, H.J.C. Yeh, S. Bulusu, Org. Magn. Reson. 12, 1 (1979)
M. Hansen, H.J. Jacobsen, Acta Chem. Scand. 26, 2151 (1972)
M.-L. Louillat, A. Biafora, F. Legros, F.W. Patureau, Angew. Chem. 53, 3505 (2014)
Acknowledgements
A.K.S. acknowledges Alexander von Humboldt foundation for a stipend and travel grants. The authors are thankful to Dr. D.N. Laikov for valuable discussion.
Author information
Authors and Affiliations
Contributions
VVS—performed analysis of the literature, optimization of geometry and quantum-chemical evaluation of spin–spin coupling constants 13C–15N for the selected compounds, prepared figures, compiled tables based on the data obtained, and also actively participated in the discussion of the results and made an oral report on this topic at the Spinus-2022 conference; AKS performed all NMR experiments and analyzed results for compound 61, participated in the discussion of the text of the manuscript, compiled Table 10, and edited the figures; VAC took an active part in the planning, writing, editing and discussion of the results, participated in statistical treatment of the result. All the authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stanishevskiy, V.V., Shestakova, A.K. & Chertkov, V.A. Analysis of 2D Maps Based on Similarity in DFT-Calculated vs Experimental 13C–15N Spin Couplings for a Representative Sample of Conformationally Rigid and Structurally Fixed Nitrogen-Containing Organic Compounds. Appl Magn Reson 53, 1693–1713 (2022). https://doi.org/10.1007/s00723-022-01503-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-022-01503-w