Abstract
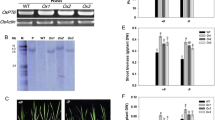
Oryza sativa PHOSPHATE RESPONSE2 (OsPHR2) can promote the uptake and use of phosphorus (P) in rice. We introduced OsPHR2 into the winter wheat (Triticum aestivum L.) variety “Zhengmai0856.” OsPHR2 was integrated into the wheat genome with two copy numbers and could be correctly transcribed and expressed. OsPHR2 was mainly expressed in the leaves at the seedling stage. From the jointing to filling stage, OsPHR2 was mainly expressed in the roots, followed by the leaves, with a low expression level in detected the tassels and stems. The transgenic lines exhibited higher P accumulation at each growth stage and increased P uptake intensity in comparison to the wild type under low P and high P conditions. Analysis of the root characteristics showed that the transgenic expression of OsPHR2 increased the maximum root length, total root length, root-to-shoot ratio, and root volume under the conditions of P deficiency or low P. A field experiment showed that the transgenic lines had a higher grain yield than the wild type under low P and high P conditions. The yield of the transgenic lines increased by 6.29% and 3.73% on average compared with the wild type under low P and high P conditions, respectively. Thus, the transgenic expression of OsPHR2 could increase P uptake and yield in wheat, but the effect was more prominent under low P conditions.







Similar content being viewed by others
References
Abel S, Nurnberger T, Ahnert V, Krauss GJ, Glund K (2000) Induction of an extracellular cyclic nucleotide phosphodiesterase as an accessory ribonucleolytic activity during phosphate starvation of cultured tomato cells. Plant Physiol 122:543–552
Abel S (2011) Phosphate sensing in root development. Curr Opin Plant Biol 14(3):303–309
Aziz T, Maqsood MA, Sabir M, Kanwal S (2011) Categorization of Brassica cultivars for phosphorus acquisition from phosphate rock on the bases of growth and ionic parameters. J Plant Nutr 34:522–533
Bari R, Pant BD, Stitt M, Scheible WR (2006) PHO2, MiR399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol 14:988–999
Bates TR, Lynch JP (2001) Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 236:243–250
Bilal HM, Aziz T, Maqsood MA, Farooq M, Yan G (2018) Categorization of wheat genotypes for phosphorus efficiency. PLoS One 13(10):e0205471
Bonser A, Lynchj P, Snapp S (1996) Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytol 132:281–288
Bustos R, Castrillo G, Linhares F, Puga MI, Rubio V, Perez-Perez J, Solano R, Leyva A, Paz-Ares J (2010) A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet 6(9):e1001102
Cheng L, Bucciarelli B, Liu J, Zinn K, Miller S, Patton-Vogt J, Allan D, Shen J, Vance CP (2011) White lupin cluster root acclimation to phosphorus deficiency and root hair development involve unique glycerophosphodiester phosphodiesterases. Plant Physiol 156:1131–1148
Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL (2006) Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 184:12–421
Chiou TJ, Lin SI (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62:185–206
Christine R, Marcel B (2002) Molecular mechanisms of phosphate transport in plants. Planta 216(1):23–27
Goldstein AH (1992) Phosphate starvation inducible enzymes and proteins in higher plants. In: Society for Experimental Biology Semi nar Series 49: Inducible Plant Proteins. Cambridge Univ. Press USA, Cambridge, p 544
Guo MN, Ruan WY, Li CY, Huang FL, Zeng M, Liu YY, Yu YN, Ding XM, Wu YR, Wu ZC, Mao CZ, Yi KK, Wu P, Mo XR (2015) Integrative comparison of the role of the PHOSPHATE RESPONSE1 subfamily in phosphate signaling and homeostasis in rice. Plant Physiol 168:1762–1776
He QJ, Wang F, Wang Y, Lu H, Yang ZL, Lv QD, Mao CZ (2019) Molecular control and genetic improvement of phosphorus use efficiency in rice. Mol Breed 39:162
He QJ, Lu H, Guo HX, Wang Y, Zhao P, Li Y, Wang F, Xu JM, Mo XR, Mao CZ (2020) OsbHLH6 interacts with OsSPX4 and regulates the phosphate-starvation response in rice. Plant J Accepted Author Manuscript 105:649–667. https://doi.org/10.1111/tpj.15061
Hou XL, Wu P, Jiao FC, Jia QJ, Chen HM, Yu J, Song XW, Yi KK (2005) Regulation of the expression of OsIPS1 and OsIPS2 in rice via systemic and local Pi signalling and hormones. Plant Cell Environ 28:353–364
Kong YZ, Wang G, Chen X, Li LY, Zhang XY, Chen ST, He YQ, Hong GJ (2021)OsPHR2 modulates phosphate starvation-induced OsMYC2 signalling and resistance to Xanthomonas oryzae pv. Oryzae. Plant Cell Environ 1-13
Li S, Wang C, Zhou L, Shou H (2014) Oxygen deficit alleviates phosphate overaccumulation toxicity in OsPHR2 overexpression plants. J Plant Res 127:433–440
Ling BQ, Bai XX, Zhou YB, Wang CX, Xu ZS, Ma YZ, Chen M, Zhang XH (2018) Functional analysis of AtTGA4 transgenic wheat tolerance to low phosphorus stress in Field. Sci Agric Sin 51(12):2225–2234
Liu F, Wang Z, Ren H, Shen C, Li Y, Ling HQ, Wu CY, Lian XM, Wu P (2010) OsSPX1 suppresses the function of OsPHR2 in the regulation of expression of OsPT2 and phosphate homeostasis in shoots of rice. Plant J 62:508–517
Liu JL, Yang L, Luan MD, Wang Y, Zhang C, Zhang B, Shi JS, Zhao FG, Lan WZ, Luan S (2015) A vacuolar phosphate transporter essential for phosphate homeostasis in Arabidopsis. Proc Natl Acad Sci 112(47):E6571–E6578
Lu RK (1998) Principles and fertilization of soil-plant nutrition. Chemical Industry Press, Beijing, China 49-53;433-443 Lynch J, Beebe S (1995) Adaptation of beans (Phaseolus vulgaris L.) to low phosphorus availability. HortScience 30(6):1165–1171
Lynch J, Beebe S (1995) Adaptation of beans (Phaseolus vulgaris L.) to low phosphorus availability. HortScience 30(6):1165–1171
Ma Q, Rengel Z, Siddique KHM (2011) Wheat and white lupin differ in root proliferation and phosphorus use efficiency under heterogeneous soil P supply. Crop Pasture Sci 62:467
Motte H, Beeckman T (2017) PHR1 balances between nutrition and immunity in plants. Dev Cell 41:5–7
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Nilsson L, Müller R, Nielsen TH (2007) Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced Phosphate uptake in Arabidopsis thaliana. Plant Cell Environ 30:1499–1512
Peng CJ, Xu WG, Hu L, Li Y, Qi XL, Wang HW, Hua X, Zhao MZ (2018) Effects of maize C4 phosphoenolpyruvate carboxylase (ZmPEPC) gene on nitrogen assimilation in transgenic wheat. Plant Growth Regul 84(1):191–205
Qi XL, Li Y, Hu L, Xu WG, Zhang JZ, Chang YY, Huang SM (2016) Greation of new transgenic wheat germplasm expressing TaPHR1 gene and studies on its biological function. Mol Plant Breed 14(12):1–11
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50:665–693
Rausch C, Bucher M (2002) Molecular mechanisms of phosphate transport in plants. Planta 216:23–37
Ren F, Guo QQ, Chang LL, Chen L, Zhao CZ, Zhong H, Li XB (2012) Brassica napus PHR1 gene encoding a MYB-like protein functions in response to phosphate starvation. PLoS One 7:e44005
Ruan WY, Guo MN, Cai LL, Hu HT, Li CY, Liu Y, Wu ZC, Mao CZ, Yi KK, Wu P, Mo XR (2015) Genetic manipulation of a high-affinity PHR1 target cis-element to improve phosphorous uptake in Oryza sativa L. Plant Mol Biol 87:429–440
Ruan W, Guo M, Wu P, Yi K (2017) Phosphate starvation induced OsPHR4 mediates Pi-signaling and homeostasis in rice. Plant Mol Biol 93:327–340
Rubio V, Linhares F, Solano R, Martin AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15:2122–2133
Secco D, Wang C, Arpat BA, Wang ZY, Poirier Y, Tyerman SD, Wu P, Shou HX, Whelan J (2012) The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol 193:842–851
Sega P, Pacak A (2019) Plant PHR transcription factors: put on a map. Genes 10:1018
Shen Q, Wen ZH, Dong Y, Li HG, Miao YX, Shen JB (2018) The responses of root morphology and phosphorus-mobilizing exudations in wheat to increasing shoot phosphorus concentration. Ann Bot Plants 10(5):1–11
Singh GT, Nielsen NE (2004) Root traits as tools for creating phosphorus efficient crop varieties. Plant Soil 260:47–57
Sun L, Song L, Zhang Y, Zheng Z, Liu D (2016) Arabidopsis PHL2 and PHR1 act redundantly as the key components of the central regulatory system controlling transcriptional responses to phosphate starvation. Plant Physiol 170:499–514
Theodorou ME, Plaxton WC (1993) Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol 101:339–344
Tian J, Wang C, Zhang Q, He X, Whelan J, Shou H (2012) Overexpression of OsPAP10a, a root-associated acid phosphatase, increased extracellular organic phosphorus utilization in rice. J Integr Plant Biol 54:631–639
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:427–447
Wang LZ, Mi GH, Chen FJ, Zhang FS (2003) Identify of winter wheat cultivars on the phosphorus nutrition efficiency. J China Agric Univ 8(5):69–73
Wang J, Sun JH, Miao J, Guo JK, Shi ZL, He MQ, Chen Y, Zhao XQ, Li B, Han FP, Tong YP Li ZS (2013) A phosphate starvation response regulator Ta-PHR1 is involved in phosphate signaling and increases grain yield in wheat. Ann Bot 111:1139–1153
Wang XM, Yi KK, Tao Y, Wang F, Wu ZC, Jiang DA, Chen X, Zhu LH, Wu P (2006) Cytokinin represses phosphate-starvation response through increasing of intracellular phosphate level. Plant Cell Environ 29:1924–1935
Wang YG (2014) The Mechanism of 0sSPX4 regulating phosphate central regulator OsPHR2 in rice. Ph.D. Zhejiang University, China
Wang Z, Zheng Z, Song L, Liu D (2018) Functional characterization of Arabidopsis PHL4 in plant response to phosphate starvation. Front Plant Sci 9:1432
Wasaki J, Yonetani R, Shinano T, Kai M, Osaki M (2003) Expression of the OsPI1 gene, cloned from rice roots using cDNA microarray, rapidly responds to phosphorus status. New Phytol 158:239–248
Yan XL, Liao H, Ge ZY, Luo XW (2000) Root architectural characteristics and phosphorus acquisition efficiency in plants. Chin Bull Bot 17(6):511–519
Zhang HF, Xu WG, Wang HW, Hu L, Li Y, Qi XL, Zhang L, Li CX, Hua X (2014) Pyramiding expression of maize genes encoding phosphoenolpyruvate carboxylase (PEPC) and pyruvate orthophosphate dikinase (PPDK) synergistically improve the photosynthetic characteristics of transgenic wheat. Protoplasma 251:1163–1173
Zhang Q, Wang C, Tian J, Li K, Shou H (2011) Identification of rice purple acid phosphatases related to phosphate starvation signalling. Plant Biol 13:7–15
Zhang L, Sun WX, Sun YF, Pei WX, Luo WZ, Sun R, Zhang ZT, Xu GH, Sun SB (2016) Roles of transcription factor gene OsPHR3 on the utilization of phosphate in rice. Chin J Rice Sci 30(4):397–405
Zheng XW, Liu C, Qiao L, Zhao JJ, Han R, Wang XL, Ge C, Zhang WY, Zhang SW, Qiao LY, Zheng J, Hao CY (2020) The MYB transcription factor TaPHR3-A1 is involved in phosphate signaling and governs yield-related traits in bread wheat (Triticum aestivum L.). J Exp Bot Accepted Author Manuscript. https://doi.org/10.1093/jxb/eraa355
Zhou J, Jiao FC, Wu ZC, Li YY, Wang XM, He XW, Zhong WQ, Wu P (2008) OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol 146(4):1673–1686
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31701510), the Genetically Modified Organisms Breeding Major Projects of China (Grant No. 2016ZX08002005-004), the Science and Technology project of Henan Province (Grant No. 192102110015), and the Science Technology Foundation for Outstanding Young Scientists of Henan Academy of Agricultural Sciences (Grant No. 2020YQ29).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that the submitted manuscript does not contain previously published material and is not under consideration for publication elsewhere. All authors listed have read the complete manuscript and have approved the submission of the paper. The manuscript is a truthful original work without fabrication, fraud, or plagiarism.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Fig. SI1
Map of the expression cassette of OsPHR2 and bar. a The map of the expression cassette of OsPHR2. b The map of the expression cassette of bar. (PNG 2960 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Fang, Y., Peng, C. et al. Transgenic expression of rice OsPHR2 increases phosphorus uptake and yield in wheat. Protoplasma 259, 1271–1282 (2022). https://doi.org/10.1007/s00709-021-01702-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01702-5