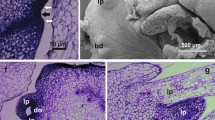
Abstract
Nodule cultures are formed through an intermediate morphogenetic route that lies between organogenesis and somatic embryogenesis. Although well described in many species, different aspects of the morphological and histological development of nodules remain to be clarified. Based on their threatened status and high ornamental value, Billbergia alfonsi-joannis and Billbergia zebrina, two epiphytic bromeliad species endemic to the South American Atlantic Forest, were studied. Nodular cultures were induced to grow from nodal segments taken from etiolated seedlings grown in vitro for 12 weeks in the dark on MS medium supplemented with 1 μM TDZ. Samples were taken for analysis weekly over 8 weeks of growth and analyzed under light, transmission electron, and scanning electron microscopes. Morphological and histological analysis showed that nodular clusters originated from stem pericycles and consisted of a polycenter, cambial tissue, cortical parenchyma, and a covering tissue. The polycenter consisted of an organizational center dispersed in parenchymal tissue. Each organizational center was formed by a vascular system surrounded by a bundle sheath. A cambial tissue surrounded these polycenters, promoting the regeneration of new nodules and leading to the formation of buds and roots. Primary nodules could generate secondary nodules in a repetitive process. Thus, histological analysis revealed the origin and formation of nodular cultures. These new data will support the establishment of micropropagation protocols and regeneration on a large scale for these species.





Similar content being viewed by others
Abbreviations
- AB:
-
Axillary bud
- BAF:
-
Billbergia alfonsi-joannis
- BZ:
-
Billbergia zebrina
- CA:
-
Cambium
- CC:
-
Cambial cell
- OC:
-
Organizational center
- LE:
-
Leaf
- LM:
-
Light microscopy
- LP:
-
Leaf primordia
- MC:
-
Meristematic cell
- MS:
-
Murashige and Skoog (1962) basal media
- NO:
-
Nodule
- NV:
-
Nodule vascularization
- PA:
-
Parenchyma
- PE:
-
Periderm
- PV:
-
Provascular cell
- RO:
-
Root
- SEM:
-
Scanning electron microscopy
- SN:
-
Secondary nodule
- ST:
-
Stem
- SNE:
-
Sieve neoelement
- TDZ:
-
Thidiazuron
- TEM:
-
Transmission electron microscopy
- TN:
-
Tracheal neoelement
- TU:
-
Tunic
- VC:
-
Vascular cylinder
References
Alves GM, Guerra MP (2001) Micropropagation for mass propagation and conservation of Vriesea friburguensis var. Paludosa Microbuds J Bromeliad Soc 51:202–212
Alves GM, Dal Vesco LL, Guerra MP (2006) Micropropagation of the Brazilian endemic bromeliad Vriesea reitzii trough nodule culture. Sci Hortic 110:204–207
Batista D, Ascensão L, Sousa MJ, Pais MS (2000) Adventitious shoot mass production of hop (Humulus lupulus L.) var. Eroica in liquid medium from organogenic nodule cultures. Plant Sci 151:47–57
Benzing DH (1980) The biology of the bromeliads. Mad River Press, USA
Brasil, Ministério do Meio Ambiente (2008) Lista Oficial das Espécies da Flora Brasileira Ameaçadas de extinção. MMA, Brasília
Carneiro LA, Araújo RFG, Brito GJM, Fonseca MHPB, Costa A, Crocomo OJ, Mansur E (1999) In vitro regeneration from leaf explants of Neoregelia cruenta (R. Graham) L.B. Smith, an endemic bromeliad from eastern Brazil. Plant Cell Tiss Org 55:79–83
Corredor-Prado JP, Schmidt EC, Guerra MP, Bouzon ZL, Dal Vesco LL, Pescador R (2015) Histodifferentiation and ultrastructure of nodular cultures from seeds of Vriesea friburgensis Mez var. Paludosa (L.B. Smith) L.B. Smith and leaf explants of Vriesea reitzii Leme & A. Costa (Bromeliaceae). J Microsc Ultrastruct. doi:10.1016/j.jmau.2015.04.001
Dal Vesco LL, Guerra MP (2010) In vitro morphogenesis and adventitious shoot mass regeneration of Vriesea reitzii from nodule cultures. Sci Hortic 125:748–755
Dal Vesco LL, Stefenon VM, Welter LJ, Scherer RF, Guerra MP (2011) Induction and scale-up of Bilbergia zebrina nodule cluster cultures: implications for mass propagation, improvement and conservation. Sci Hortic 128:515–522
Evert RF (2006) Esau’s plant anatomy. Meristems, cells, and tissues of the plant body: their structure, function, and development. Wiley, Hoboken
Fay MF (1992) Conservation of rare and endangered plants using in vitro methods. In Vitro Cell Dev B 28:l–4
Fermino Júnior PCP, Lando AP, Santos M, Pescador R (2014) Morfo-histologia de culturas nodulares na micropropagação de Aechmea setigera Mart. Ex Schult. & Schult. F. (Bromeliaceae). Evid 14:85–98
Ferreira S, Batista D, Serrazina S, Pais MS (2009) Morphogenesis induction and organogenic nodule differentiation in Populus euphratica Oliv. Leaf explants. Plant Cell Tiss Org 96:35–43
Fortes AM, Pais MS (2000) Organogenesis from internode-derived nodules of Humulus lupulus var. Nugget (Cannabinaceae): histological studies and changes in the starch content. Am J Bot 87:971–979
George EF (1993) Plant propagation by tissue culture: the technology. Exegetics, Edington
Gerrits PO, Smid L (1983) A new, less toxic polymerization system for the embedding of soft tissues in glycol methacrylate and subsequent preparing of serial sections. J Microsc 132:81–85
Guerra MP, Dal Vesco LL (2010) Strategies for the micropropagation of bromeliads. In: Jain SM, Ochatt SJ (eds) Protocols for in vitro propagation of ornamental plants: methods in molecular biology. Humana Press-Springer, New York, pp 47–66
Haensch KT (2004) Morphohistological study of somatic embryolike structures in hypocotyl cultures of Pelargonium x hortorum Bailey. Plant Cell Rep 22:376–381
Horridge GA, Tamm SL (1969) Critical point drying for scanning electron microscopy study of ciliary motion. Science 163:817–818
Krauss BH (1948) Anatomy of the vegetative organs of the pineapple, Ananas comosus (L.) Merr. I. Introduction, organography, the stem, and the lateral branch or axillary buds. Bot Gaz 110:159–217
McConnell JR, Barton MK (1998) Leaf polarity and meristem formation in Arabidopsis. Development 125:2935–2942
McCown BH, Zeldin EL, Pinkalla HÁ, Dedolph RR (1988) Nodule culture: a developmental pathway with high potencial for regeneration, automated micropropagation, and plant metabolite production from woody plants. In: Hanover JH, Keathley DE (eds) Genetic manipulation of woody plants. Plenum, New York, pp 149–166
Menezes NL, Silva DC, Arruda RCO, Melo-de-Pinna GF, Cardoso VA, Castro NM, Scatena VL, Scremin-Dias E (2005) Meristematic activity of the endodermis and the pericycle in the primary thickening in monocotyledons. Considerations on the “PTM”. An Acad Bras Cienc 77:259–274
Morel GM, Wetmore RH (1951) Tissue culture of monocotyledons. Am J Bot 38:138–140
Moyo M, Finnie JF, van Staden J (2009) In vitro morphogenesis of organogenic nodules derived from Sclerocarya birrea subsp. Caffra leaf explants. Plant Cell Tiss Org 98:273–280
Murashige T, Skoog F (1962) A revised medium for rapid growth and biossays with tobacco tissue cultures. Physiol Plant 15:473–497
Ntui VO, Azadi P, Supaporn H, Mii M (2010) Plant regeneration from stem segment-derived friable callus of “Fonio” (Digitaria exilis (L.) Stapf.). Sci Hortic 125:494–499
O’Brien TP, McCully ME (1981) The study of plant structure: principles and selected methods. Termarcarphy Pty, Melburne
O’Brien TP, Feder N, M’ccully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–73
Ogura Y (1972) Comparative anatomy of vegetative organs of the pteridophytes. Gebrüder Borntraeger, Berlin
Piéron S, Belaizi M, Boxus P (1993) Nodule culture, a possible morphogenetic pathway in Chicorium intybus L. propagation. Sci Hortic 53:1–11
Piéron S, Boxus P, Dekegel D (1998) Histological study of nodule morphogenesis from Chicorium intybus L. leaves cultivated in vitro. In vitro Cell Dev Biol Plant 34:87–93
Reitz R (1983) Bromeliáceas e a malária—bromélia endêmica. Herbário Barbosa Rodrigues, Itajaí
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–2012
Salaj J, Petrovska B, Obert B, Pret’ova A (2005) Histological study of embryo-like structures initiated from hypocotyls segments of flax (Linum usitatissimum L.). Plant Cell Rep 24:590–595
Scherer RF, Garcia AC, Fraga HPF, Dal Vesco LL, Steinmacher DA, Guerra MP (2013) Nodule cluster cultures and temporary immersion bioreactors as a high performance micropropagation strategy in pineapple (Ananas comosus var. comosus). Sci Hortic 151:38–45
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Teng WL (1997) An alternative propagation method of Ananas comosus trough nodule culture. Plant Cell Rep 16:454–457
Warrag E, Lesney MS, Rockwood DJ (1991) Nodule culture and regeneration of Eucalyptus grandis hybrids. Plant Cell Rep 9:586–589
Woo SM, Wetzstein HY (2008) Morphological and histological evaluations of in vitro regeneration in Elliottia racemosa leaf explants induced on media with thidiazuron. J Amer Soc Hort Sci 133:167–172
Acknowledgments
This work was supported by the National Council for Scientific and Technological Development (CNPq, Brazil) and Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil). The authors acknowledge the staff of the Central Laboratory of Electron Microscopy (LCME), Plant Anatomy Laboratory (LAVEG), and Physiology Laboratory of Plant Development and Genetics (LFDGV) of the Federal University of Santa Catarina, Brazil.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
de Souza, T.V., Thiesen, J.F., Lando, A.P. et al. Morpho-histodifferentiation of Billbergia Thunb. (Bromeliaceae) nodular cultures. Protoplasma 254, 435–443 (2017). https://doi.org/10.1007/s00709-016-0962-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-0962-2