Abstract
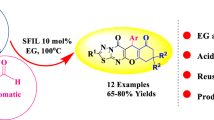
Herein, we report the green synthesis of a new series of benzo[d]imidazo[2,1-b]thiazole-1-ium hydroxide derivatives by a one-pot, three-component reaction of arylglyoxals, quinoline-2,4-diol, and 2-aminobenzothiazole in the presence of TEA/AcOH (1:2) as a catalyst at 50 °C in water, which appears as a green solvent. The structures of all 12 examples were confirmed by 1H NMR, 13C NMR, mass spectral data, and FT-IR. This procedure provides mild reaction conditions, using green solvent, high yields, easy work-up and simple isolation of structurally divers tricyclic benzo[d]imidazo[2,1-b]thiazole derivatives which may have biological and pharmaceutical activities.
Graphical abstract


Similar content being viewed by others
References
Sultana F, Bonam SR, Reddy VG, Nayak VL, Akunuri R, Routhu SR, Alarifi A, Halmuthur MSK, Kamal A (2018) Bioorg Chem 76:1
Ziyaei Halimehjani A, Hosseinkhany S (2015) Synthesis 47:3147
Kumbhare RM, Nagragu C (2011) Lett Drug Design Discovery 8:633
Katritzky AR, Wu J, Rachwal S, Rachwal B, Macomber DW, Smith TP (1992) Org Prep Proced Int 24:463
Dauben P, Dodd RH (2000) Org Lett 2:2327
McReynolds MD, Dougherty JM, Hanson PR (2004) Chem Rev 104:2239
Khatri CK, Mali AS, Chaturbhuj GU (2017) Monatsh Chem 148:1463
Farahi M, Karami B, Banaki Z, Rastgoo F, Eskandari K (2017) Monatsh Chem 148:1469
Mamaghani M, Sheykhan M, Sadeghpour M, Tavakoli F (2018) Monatsh Chem 149:1437
Ghaffari Khaligh N, Mihankhah T, Rafie Johan M, Ching JJ (2018) Monatsh Chem 149:1083
Soleiman-Beigi M, Mohammadi F (2017) Monatsh Chem 148:2123
Fogg DE, Santos EN (2004) Coord Chem Rev 248:2365
Ajamian A, Gleason JL (2004) Angew Chem Int Ed 43:3754
Seigal BA, Fajardo C, Snapper ML (2005) J Am Chem Soc 127:16329
Parik N, Roy SR, Seth K, Kumar A, Chakraborti AK (2016) Synthesis 48:547
Chanda A, Fokin VV (2009) Chem Rev 109:725
Premakumari C, Muralikrishna A, Padmaja A, Padmavathi V, Park SJ, Kim T-J, Reddy GD (2014) Arabian J Chem 7:385
Eftekhari-Sis B, Zirak M, Akbari A (2013) Chem Rev 113:2958
Soural M, Bouillon I, Krchnak V (2008) J Comb Chem 10:923
Premakumari C, Muralikrishna A, Padmaja A, Padmavathi V, Park SJ, Kim TJ, Reddy GD (2014) Arabian J Chem 7:385
Combs DW, Rampulla MS, Bell SC, Klaubert DH, Tobia AJ, Falotico R, Haertlein B, Lakas-Weiss C, Moore JB (1990) J Med Chem 33:380
Sotelo E, Coelho A, Ravina E (2003) Tetrahedron Lett 44:4459
Ji X-M, Xu L, Yan Y, Chen F, Tang R-Y (2016) Synthesis 48:687
Park JH, El-Gamal MI, Lee YS, Oh CH (2011) Eur J Med Chem 46:5769
Furlan A, Colombo F, Kover A, Issaly N (2012) Eur J Med Chem 47:239
Andreani A, Granaiola M, Locatelli A, Morigi RJ (2012) Med Chem 55:2078
Fidanze SD, Erickson SA, Wang GT, Mantei R, Clark RF, Sorensen BK, Bamaung NY, Kovar P, Johnson EF, Swinger KK, Stewart KD, Zhang Q, Tucker LA, Pappano WN, Wilsbacher JL, Wang J, Sheppard GS, Bell RL, Davidsen SK, Hubbard RD (2010) J Med Chem 20:2452
Kamal A, Sultana F, Ramaiah MJ, Srikanth YVV, Viswanath A, Kishor C, Sharma P, Pushpavalli SNCVL, Addlagatta A, Pal-Bhadra M (2012) Chem Med Chem 7:292
Gali R, Banothu J, Porika M, Velpula R, Bavantula R, Abbagani S (2014) RSC Adv 4:53812
Malik JK, Soni H, Singhai AK (2013) J Pharm Res 7:39
Guzeldemirci NU, Kucukbasmacı O (2010) Eur J Org Chem 45:63
Palkar M, Noolvi M, Sankangoud R, Maddi V (2010) Arch Pharm Chem Life Sci 343:353
Al-Tel TH, Al-Qawasmeh RA, Zaarour R (2011) Eur J Med Chem 46:1874
Farag AM, Mayhoub AS, Barakat SE, Bayomi AH (2008) Bioorg Med Chem 16:4569
Guzeldemirci NU, Kucukbasmaci O (2010) Eur J Med Chem 45:63
Ager IR, Barnes AC, Danswan GW, Hairsine PW (1988) J Med Chem 31:1098
Gowen BB, Bray M (2011) Future Microbiol 6:1429
Barradas JS, Errea MI, D-Accorso NB, Sepulveda CS, Damonte EB (2011) Eur J Med Chem 46:259
Andreani A, Burnelli S, Granaiola M, Guardigli M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Rizzoli M, Varoli L, Roda A (2008) Eur J Org Chem 43:657
Terzioglu N, Van Rijn RM, Bakker RA, De Esch IJP, Leurs R (2004) Bioorg Med Chem Lett 14:5251
Andreani A, Leoni A, Locatelli A, Morigi R, Rambaldi M, Cervellati R, Greco E, Kondratyuk TP, Park EJ, Huang K, Van BRB, Pezzuto JM (2013) Eur J Med Chem 68:412
Andreani A, Rambaldi M, Leoni A, Locatelli A, Andreani F, Gehret JC (1996) Pharm Acta Helv 71:247
Christodoulou MS, Colombo F, Passarella D, Ieronimo G (2011) Bioorg Med Chem 19:1649
Blackburn C, Duffey MO, Gould AE, Kulkarni B (2010) Bioorg Med Chem Lett 20:4795
Yao R, Yasuoka A, Kamei A, Kitagawa Y (2010) J Agric Food Chem 58:2168
Yousefi BH, Manook A, Drzezga A, Reuter BV, Schwaiger M, Wester HJ, Henriksen G (2011) J Med Chem 54:949
Alagille D, DaCosta H, Baldwin RM, Tamagnan GD (2011) Bioorg Med Chem Lett 21:2966
Xie Y, Chen X, Wang Z, Huang H, Yi B, Deng G (2017) J Green Chem 19:4294
Roslan II, Ng KH, Chuah GK, Jaenicke S (2017) Beilstein J Org Chem 13:2739
Balwe SG, Jeong Y (2016) RSC Adv 6:107225
Balwe SG, Lim KT, Cho BG, Jeong YT (2017) Tetrahedron 73:3564
Khalafy J, Etivand N, Dilmaghani S, Ezzati M, Poursattar Marjani A (2014) Tetrahedron Lett 55:3781
Khalafy J, Mohammadlou M, Mahmoody M, Salami F, Poursattar Marjani A (2015) Tetrahedron Lett 56:1528
Poursattar Marjani A, Khalafy J, Salami F, Mohammadlou M (2015) Synthesis 47:1656
Poursattar Marjani A, Khalafy J, Salami F, Ezzati M (2015) Arkivoc 5:277
Poursattar Marjani A, Khalafy J, Mahmoodi S (2016) Arkivoc 3:262
Ezzati M, Khalafy J, Poursattar Marjani A, Prager RH (2017) Tetrahedron 73:6587
Khalafy J, Majidi Arlan F, Soleimani Chalanchi S (2018) J Heterocycl Chem 55:149
Ezzati M, Khalafy J, Marjani AP, Prager RH (2018) Aust J Chem 71:435
Javahershenas R, Khalafy J (2018) Heterocycl Commun 24:37
Khalafy J, Ilkhanizadeh S, Ranjbar M (2018) J Heterocycl Chem 55:951
Riley HA, Gray AR (1943) In: Blatt AH (ed) Organic syntheses, collective vol II. Wiley, New York, pp 509–510
Baldwin JE (1976) J Chem Soc, Chem Commun 18:734
Acknowledgements
The authors gratefully acknowledge the financial assistance from Urmia University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Etivand, N., Ahmadi Sabegh, M. & Khalafy, J. Synthesis of a new series of benzo[d]imidazo[2,1-b]thiazole-1-ium hydroxides by a one-pot, three-component reaction in water. Monatsh Chem 150, 317–325 (2019). https://doi.org/10.1007/s00706-018-2315-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2315-7