Abstract
A stereoselective total synthesis of macrodilactone, verbalactone is reported. The strategy utilizes an iterative Jacobsen hydrolytic kinetic resolution, nucleophilic ring opening, and macrolactonisation under Yamaguchi reaction conditions as the key steps.
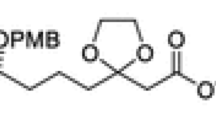
Graphical abstract


Similar content being viewed by others
References
Baccharin B, Still CW, Gennari C, Noguez JA, Pearson DA (1984) J Am Chem Soc 106:261
Nozoe S, Hirai K, Tsuda K, Ishibashi K, Shirasaka M (1965) Tetrahedron Lett 6:4675
Krohn K, Farooq U, Florke U, Schulz B, Draeger S, Pescitelli G, Salvadori P, Antus S, Kurtan T (2007) Eur J Org Chem 2007:3206
Noda A, Aoyagi S, Machinaga N, Kibayashi C (1994) Tetrahedron Lett 35:8237
MacMillan J, Simpson TJ (1973) J Chem Soc Perkin 1:1487
Kis Z, Furger P, Sigg HP (1969) Experientia 25:123
Yadav JS, Subba Reddy UV, Subba Reddy BV (2009) Tetrahedron Lett 50:5984
Lee DM, Kang HY (2008) Bull Korean Chem Soc 29:1671
Kind R, Zeeck A, Grabley S, Thiericke R, Zerlin M (1996) J Nat Prod 59:539
Ghisalberti EL, Hargreaves JR, Skelton BW, White AH (2002) Aust J Chem 55:233
Magiatis P, Spanakis D, Mitaku S, Tsitsa E, Mentis A, Harvala C (2001) J Nat Prod 64:1093
Doshida J, Hasegawa H, Onuki H, Shimidzu N (1996) J Antibiot 49:1105
Takano S, Seton M, Ogasawara K (1992) Tetrahedron Asymmetry 3:533
Bennett F, Knight D, Fenton G (1991) J Chem Soc Perkin Trans 1:1543
Bennett F, Knight D, Fenton G (1989) Heterocycles 29:639
Yang YL, Falck JR (1982) Tetrahedron Lett 23:4305
Gogoi S, Barua NC, Kalita B (2004) Tetraheron Lett 45:5577
Sharma GVM, Reddy CG (2004) Tetraheron Lett 45:7483
Florent A, Marie-Cecile L, Janine C (2007) Synlett 18:451
Salunke GB, Shivakumar I, Gurjar MK (2009) Tetrahedron Lett 50:2048
Garg A, Singh VK (2009) Tetraheron 65:8677
Das B, Laxmminarayana K, Krishnaiah M, Nandan Kumar D (2009) Helv Chim Acta 92:1840
Sabitha G, Bhaskar V, Yadav JS (2008) Synth Commun 38:3129
Wu JZ, Gao J, Ren GB, Zhen ZB, Zhang YH, Wu YK (2009) Tetrahedron 65:289
Alluraiah G, Sreenivasulu R, Murthy IS, Raju RR (2014) Monatsh Chem 145:2019
Dias Luiz C, Meira Paulo RR (2005) J Org Chem 70:4762
Lescure P, Huet F (1987) Synthesis 1987:404
Schaus SE, Brandes BD, Larrow JF, Tokunaga M, Hansen KB, Gould AE, Furrow ME, Jacobsen EN (2002) J Am Chem Soc 124:1307
Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M (1979) Bull Chem Soc Jpn 52:1989
Acknowledgments
We are grateful to JNTU for constant encouragement during this research program and GVK BIO, Hyderabad for providing analytical facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madala, M., Raman, B., Sastry, K.V. et al. A concise stereoselective total synthesis of verbalactone. Monatsh Chem 147, 1985–1990 (2016). https://doi.org/10.1007/s00706-016-1682-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1682-1