Abstract
Background
Sex-related differences in patients with aneurysmal subarachnoid hemorrhage (aSAH) exist. More females than males are affected. Aneurysm location is associated to sex. The relationship between sex and outcome, however, is unclear. Possible differences in management might influence the occurrence of primary and secondary brain injury and thus outcome. The study compares demographics, intensity of treatment, complications, and outcome among females and males with aSAH.
Methods
All consecutive patients with aSAH admitted to the neurocritical care unit, University Hospital Zurich over a 5-year period were eligible in this retrospective study. Patients’ characteristics, comorbidities, aSAH severity, frequency of vasospasm/delayed cerebral ischemia, frequency of invasive interventions, and 3-month outcome were compared by sex. Univariate analysis was performed with the data dichotomized by sex, and outcome. Multivariate analysis for prediction of outcomes was performed.
Results
Three hundred forty-eight patients were enrolled (64% females). Women were older than men. Comorbidities, scores at admission, and treatment modality were comparable among males and females. Vasospasm and DCI occurred similarly among females and males. Interventions and frequency of intraarterial spasmolysis were comparable between sexes. In the multivariate analysis, increasing age, female sex, increasing comorbidities, WFNS and Fisher grade, and presence of delayed cerebral ischemia were predictors of unfavorable outcome when considering all patients. However, after excluding death as a possible outcome, sex did not remain a predictor of unfavorable outcome.
Conclusions
In the study population, women with aSAH might have present a worse outcome at 3 months. However, no differences by sex that might explain this difference were found in intensity of treatment and management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sex-related differences in patients with aneurysmal subarachnoid hemorrhage (aSAH) exist [13]. Generally, females are more likely to suffer from aSAH [10, 24, 28, 30, 35]. Possible reasons discussed are the intrinsic weakness of the vessel walls, collagen, and elastin interference factors, as well as hormonal influence that may play a role in aneurysm formation in women [17, 31]. Furthermore, some risk factors (e.g., smoking) increase the odds of aneurysm rupture to a greater extent in women [12, 20, 21]. Aneurysm location itself also appears to be associated to sex. Most aneurysms in females are located along the internal carotid artery while in males, they are located along the anterior cerebral artery [1, 16]. Hemodynamic factors in relation to sex-specific cerebral vascular anatomy might be the reason for these sex-related differences in aneurysm location [22]. The relationship between sex and vasospasm as well as delayed cerebral ischemia (DCI), however, is poorly characterized. The available data on sex hormones and vasospasm/DCI are contradicting [4, 9, 15, 34]. Similarly, data regarding the effect of sex and gender on clinical course, intensity of treatment, and outcome are controversial [3, 11, 23]. Potential sex-related differences in patients with aSAH might arise during the hospital course. These differences might have an impact on disease progression. We previously reported that male patients with spontaneous intracerebral hemorrhage were almost three times as likely to receive an external ventricular drain (EVD) in comparison to women in the absence of radiographic or clinical differences. We suggested that a form of “gender-bias” might have influenced the decision-making process for the insertion of an EVD [33]
The identification of sex- and gender-related differences in patients with aSAH is of great interest to evaluate the current monitoring and treatment strategies in order to offer a more personalized management and to better distribute the available resources. The aim of this study was to compare clinical characteristics, management, intensity of treatment, and outcome among males and females with aSAH.
Methods
Patient population, inclusion and exclusion criteria
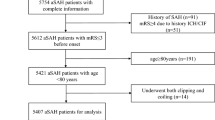
We retrospectively reviewed the medical records of all consecutive patients with aSAH admitted to the neurocritical care unit (NCCU) of the University Hospital Zurich between January 2016 and December 2021. The only patients included were adults (≥ 18 years old) with aSAH (i.e., with imaging evidence of a ruptured aneurysm). Exclusion criteria were (1) patients with only unruptured, traumatic, fusiform, dissecting, or mycotic aneurysms; (2) patient’s written or documented oral refusal to have their data analyzed for research projects. The local ethic committee approved the study. It was performed in accordance with the ethical standards as laid down in the 2013 Declaration of Helsinki. STROBE guidelines were used to draft the manuscript.
Patients’ management
Aneurysm securing
Based on our institutional protocol, aneurysm repair occurs within 24 h from bleeding by surgical or endovascular means. The decision for surgical or endovascular treatment is taken by the neurosurgeons and the neuroradiologist in charge.
External ventricular drain (EVD; BACTISEAL® EVD Catheter, CODMAN, Johnson & Johnson, Raynham, MA, USA)
The decision for the insertion of an EVD is taken by the treating neurosurgeon (at times in consultation with the neuroradiologists) using the following criteria: (1) In case of enlargement of the third ventricle and of the temporal horns of the lateral ventricles (i.e., ventriculomegaly with signs of acute occlusive hydrocephalus), (2) in case direct monitoring of the intracranial pressure (ICP) is essential such as in unconscious to comatose patients. In these patients, EVD is inserted within the first hours after admission.
Invasive multimodal neuromonitoring
In case of impaired consciousness either due to the severity of disease itself or due to the need for deep sedation (e.g., issues with ICP or artificial ventilation), beyond ICP-monitoring, probes for multimodal neuromonitoring before the vasospasm phase are inserted mostly bilateral into the frontal lobes. The invasive multimodal neuromonitoring includes hourly evaluated cerebral microdialysis (evaluating glucose, lactate and pyruvate levels; CMA 70, CMA Mikrodialysis, Solna, Sweden), continuous measurement of brain tissue oxygenation, and depending on the depth of sedation or previous seizures/seizure risks continuous electroencephalography. The decision for the insertion of invasive multimodal neuromonitoring is taken by the neurocritical care specialists and the treating neurosurgeons.
During the vasospasm phase (at least until day 14 after aSAH), all patients (irrespective of initial clinical or radiologic grade) are treated at the NCCU. For earliest possible detection of delayed cerebral ischemia (DCI) and vasospasm, serial assessment of neurological function (up to hourly depending on clinical state) and daily transcranial Doppler measurements are performed. Nimodipine is administered to all patients either orally or intravenously for the prevention of vasospasm using standardized dosages. In patients with either clinical deterioration or worsening of invasive neuromonitoring values, CT scans (including angiography and perfusion imaging) are performed (available at all time).
Intraarterial spasmolysis
In case of symptomatic vasospasm confirmed by CT-angiography with corresponding clinical deterioration or corresponding perfusion deficit upon perfusion imaging that are refractory to hemodynamic therapy, intraarterial spasmolysis using nimodipine is performed (available during the whole week 24 h per day). The decision for the performance of an intraarterial spasmolysis is taken inter-disciplinary by the neurocritical care specialist, the neuroradiologist, and the neurosurgeon.
Data collection
Data collection was performed by scanning the electronic health records for demographic characteristics, clinical and radiological information (Fisher grade, location of ruptured aneurysm, presence of intracerebral as well as intraventricular or subdural hemorrhage including location, and presence of ventriculomegaly, presence of compressed basal cisterns), treatment modality (clipping, coiling or flow-diverter based therapy), clinical course (occurrence of ventriculostomy-related infection, vasospasm, or DCI), and outcome data. The radiological findings aside from DCI as well as vasospasm were extracted from initial CT (including CT-angiography) imaging. DCI was defined as a cerebral infarction on CT or MRI excluding other causes (such as endovascular treatment or clipping) not on the CT scan 24–48 h after aneurysm occlusion [19], excluding the clinical correlate of DCI (occurrence of focal neurological impairment or decrease of 2 or more Glasgow Coma Scale (GCS) points for at least 1 h) to reduce the bias caused by the retrospective design of this study. The diagnosis of vasospasm was only used for cases with respective description on CT, MR, or digital subtraction angiography. The Charlson Comorbidity Index (CCI) was assessed to evaluate relevant comorbidities [7]. Outcome is presented using the Glasgow Outcome Scale Extended (GOSE) extracted from routine follow-up consultations at 3 months (which include a neurological examination, as well as a description of current occupation including the percentage of working capability). A dichotomized GOSE in favorable (GOSE 5–8) and unfavorable (GOSE 1 to 4) was considered in the analysis, as in previous studies [5, 32]. Furthermore, we analyzed separately patients who died (GOSE 1) and survivors. These were further dichotomized in patients with GOSE 2–4 (survivors with unfavorable outcomes) versus patients with excellent outcome (GOSE 7–8).
Statistical analysis
Statistical analysis was performed using SPSS version 26. Data was always dichotomized by sex (male vs. female) or depending on outcome. Descriptive statistics are reported as counts/percentages, mean ± standard deviation, or as median including the interquartile range as appropriate. All continuous data were tested for normality using Shapiro–Wilk’s test. Categorical variables are compared with Pearson’s χ2 or Fisher’s exact test, continuous/ordinal variables using Student’s t-tests or Mann–Whitney U tests for parametric and non-parametric data, respectively, where appropriate.
Univariate logistic regression permitted to find predictive variables associated with either outcome group: unfavorable outcome (GOSE 1 to 4), or death (GOSE 1), survivors with unfavorable outcomes (GOSE 2–4) or excellent outcome (GOSE 7–8) by a P value < 0.05 and entered in multivariable logistic regression models. Due to collinearity of the specific imaging features (i.e., intraventricular hemorrhage, ventriculomegaly etc.) and the Fisher grade, they were left out of the multivariate model. Furthermore, we excluded the interventions from the multivariate analysis as these are performed depending on clear criteria and thus inadequate for outcome prediction. The odds ratios (ORs) were calculated and expressed with the corresponding 95% confidence intervals (95% CI). All tests were performed two-sided and a P value ˂0.05 was considered statistical significant.
Results
Overall, 348 patients with SAH met the inclusion criteria. The majority of patients were female (N = 222, 64%). Patients’ demographics, comorbidities, and severity of aSAH at admission are presented in Table 1. Women were older than men (p = 0.001). No differences in presence of comorbidities, as well as scores at admission, were found among females and males. More men than women had an aneurysm of the anterior communicating artery (p = 0.0002), while more women than men had an aneurysm of the posterior communicating artery (p = 0.017) (Table 1). Radiological findings, including presence of intracerebral hemorrhage, intraventricular hemorrhage, subdural hematoma, ventriculomegaly, and blood in the basal cisterns, were comparable among men and women, as shown in Table 2.
No differences by sex in treatment modality to aneurysm repair were found, as presented in Table 2, as well as in frequency of insertion of EVD, lumbar drain, and multimodal neuromonitoring. Vasospasm occurred similarly among men and women, as well as DCI, as shown in Table 2. An intraarterial spasmolysis was performed similarly among men and women, as shown in Table 2.
Length of ICU-stay was similar among men and women, as reported in Table 2. Data on GOSE at 3 months are presented in Table 2 and Fig. 1. An unfavorable outcome was more frequently in females than males (50.5 vs. 36.5%, p = 0.013). The findings were similar after stratification of the patients by severity based on the Fisher scale (grade 1–2 vs. 3–4), and after stratification by clinical neurological presentation based on WFNS grading system (WFNS 1–3 vs. WFNS 4–5) (data not shown).
Frequency of withdrawal of life-sustaining therapies as well as of immediate palliation was comparable among women and men, as shown in Table 2.
Based on the results of the univariate analysis evaluating factors associated with unfavorable outcome (GOSE 1–4), death (GOSE1), survivors with unfavorable outcome (GOSE 2–4), and excellent outcome (GOSE 7 and 8), multivariate analysis was performed (Table 3). Increasing age, female sex, increasing CCI, increasing WFNS, and Fisher, as well as presence of DCI, were independently associated with unfavorable outcome. When considering only survivors, sex lost its predictive value. Increasing age, increasing WFNS, and Fisher scores were predictors of death. Lower Charlson Comorbidity index and WFNS score were predictors of excellent outcomes.
Discussion
This study investigates the role of sex on management in patients with aSAH. The strength of this study lies in the use of a database of consecutive patients with aSAH during a 5-year period with several parameters collected and no missing data. Similarly to previous findings, in the study population, more females than males were affected by aSAH [11, 27, 30]. Several reasons could explain this female prevalence, including hormonal influences on vascular remodeling and potentially increased frequency of vessel wall weakness and risk of aneurysmal rupture in females [13]. With regard to aneurysm securing strategies, management, and frequency of complications, no differences by sex were found in the study population.
Previous studies, particularly in the field of cardiology, reported that women are less likely to receive evidence-based acute treatments for acute coronary syndrome than men [6, 18]. A form of gender bias — mostly unconscious — was the presumed reason for less aggressive treatment for women. Similarly, our group recently reported that at our institution women with spontaneous intracerebral hemorrhage are less likely than men to receive an EVD despite comparable frequency of hydrocephalus/ventriculomegaly. Multivariate analysis proved male gender to be independently associated with EVD insertion in these patients even after correction for location and severity of bleeding [33].
At our NCCU, patients with aSAH are treated based on a standardized protocol, which includes early aneurysm securing, ICP-monitoring in case of severely decreased vigilance, EVD insertion in case of ventriculomegaly or clinical signs for increased ICP, as well as serial TCCD measurements and insertion of multimodal neuromonitoring for the early detection of symptomatic vasospasm and DCI. Decisions are taken in multidisciplinary consensus between neurosurgeons, neuroradiologists, and neurocritical care specialists. This protocol based, inter-disciplinary approach may minimize the influence of “gender bias”[26].
With regard to outcome, previous findings in patients with aSAH are inconclusive [13]. In our study population, women fared worse than men did. We suppose that this result might be partly due to the older age of the females. In fact it is well-known that older age is a determinant for poor outcome in patients with aSAH [8, 25]. In the multivariate analysis, increasing age is independently associated with unfavorable outcome. Furthermore, female sex per se was a predictor of unfavorable outcome if including death as a possible outcome, confirming the recent preliminary data from a large cohort (n > 8000 patients) of aSAH patients [2]. This finding is quite interesting and it might also be secondary to the treatment targets that we set without taking into account patients’ sex. For instance, the blood pressure values targeted are different in presence of vasospasm. In these cases, controlled arterial hypertension is induced to maintain/reach sufficient cerebral blood flow stable to prevent or treat DCI. However, induced hypertension might lead to serious adverse events, as cardiac arrhythmia, myocardial infarction, pulmonary edema, brain edema, hemorrhagic infarction, and rebleeding [14]. Because sex-related differences in cardiovascular conditions exist [29], these should be considered in the management of patients with SAH. Previous studies on clinical outcomes following aSAH and sex are not conclusive [13], probably also due to methodological issues on outcome assessment, which complicate comparison of findings across studies. To improve understanding of the role sex plays in this context, it is imperative that outcomes studies include sex as a predictor variable in their analyses and examine sex-specific effects of interventions.
The findings that increasing age, increasing WFNS, and Fisher scores are predictors of death after aSAH are not new. Interestingly, despite a trend for more excellent outcome in females than males, this finding was not confirmed in the multivariate analysis.
Limitations
The study has some limitations. Firstly, this is the experience of a single-center, limiting the generalizability of our findings. Secondly, due to the retrospective nature of the study, we cannot exclude detection and referral biases. Thirdly, despite the large number of parameters collected, some outcome of interest as frequency of cardiovascular complications during the NCCU-stay are not available, permitting only speculations on the reported differences in outcome by sex. Fourthly, we can only make some speculations on the reasons of the worse outcome for females compared to males. Some well-known determinants of poor outcomes (for instance, aneurysmal re-rupture, diffuse brain swelling on CT scan, and fever) in patients with aSAH were not taken into account in the analysis. Finally, outcome was assessed only 3 months following the SAH, limiting the evaluation of a possible neurological improvement later on.
Conclusion
No differences in the frequency of invasive therapies between the sexes could be found in this study. While this study did not directly evaluate the effect of using a protocol, the results, particularly if considering the previous published findings referring to patients with spontaneous intracerebral hemorrhage, suggest that a well-established inter-disciplinary decision-making process might minimize sex-related bias in treatment. The results also implicate that beyond age, there might be more subtle differences or even treatment-related complications that might have caused the difference in outcome that will have to be taken into account in future prospective studies.
References
Aarhus M, Helland CA, Wester K (2009) Differences in anatomical distribution, gender, and sidedness between ruptured and unruptured intracranial aneurysms in a defined patient population. Acta Neurochir (Wien) 151:1569–1574. https://doi.org/10.1007/s00701-009-0316-3
Anadani M, Kumar A, Phuah C-l, De Havenon AH, MacDonald R, Trialists SHI (2021) Abstract P434: Sex differences in outcomes after subarachnoid hemorrhage: insight from the subarachnoid hemorrhage international trialists repository. Stroke 52:AP434
Ayala C, Croft JB, Greenlund KJ, Keenan NL, Donehoo RS, Malarcher AM, Mensah GA (2002) Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995–1998. Stroke 33:1197–1201. https://doi.org/10.1161/01.str.0000015028.52771.d1
Barrow JW, Turan N, Wangmo P, Roy AK, Pradilla G (2018) The role of inflammation and potential use of sex steroids in intracranial aneurysms and subarachnoid hemorrhage. Surg Neurol Int 9:150. https://doi.org/10.4103/sni.sni_88_18
Bogli SY, Wang S, Romaguera N, Schutz V, Rafi O, Gilone M, Keller E, Imbach LL, Brandi G (2022) Impact of seizures and status epilepticus on outcome in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 36(3):751–759. https://doi.org/10.1007/s12028-022-01489-0
Bugiardini R, Manfrini O, Majstorovic Stakic M, Cenko E, Boytsov S, Merkely B, Becker D, Dilic M, Vasiljevic Z, Koller A, Badimon L (2014) Exploring in-hospital death from myocardial infarction in Eastern Europe: from the International Registry of Acute Coronary Syndromes in Transitional Countries (ISACS-TC); on the Behalf of the Working Group on Coronary Pathophysiology & Microcirculation of the European Society of Cardiology. Curr Vasc Pharmacol 12:903–909. https://doi.org/10.2174/157016111206141210122150
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P, American Heart Association Stroke C, Council on Cardiovascular R Intervention, Council on Cardiovascular N, Council on Cardiovascular S Anesthesia, Council on Clinical C (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 43:1711–1737. https://doi.org/10.1161/STR.0b013e3182587839
Crago EA, Sherwood PR, Bender C, Balzer J, Ren D, Poloyac SM (2015) Plasma estrogen levels are associated with severity of injury and outcomes after aneurysmal subarachnoid hemorrhage. Biol Res Nurs 17:558–566. https://doi.org/10.1177/1099800414561632
Cras TY, Bos D, Ikram MA, Vergouwen MDI, Dippel DWJ, Voortman T, Adams HHH, Vernooij MW, Roozenbeek B (2020) Determinants of the presence and size of intracranial aneurysms in the general population: the Rotterdam Study. Stroke 51:2103–2110. https://doi.org/10.1161/STROKEAHA.120.029296
De Marchis GM, Schaad C, Fung C, Beck J, Gralla J, Takala J, Jakob SM (2017) Gender-related differences in aneurysmal subarachnoid hemorrhage: a hospital based study. Clin Neurol Neurosurg 157:82–87. https://doi.org/10.1016/j.clineuro.2017.04.009
Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, Anderson CS (2005) Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 36:2773–2780. https://doi.org/10.1161/01.STR.0000190838.02954.e8
Fuentes AM, Stone McGuire L, Amin-Hanjani S (2022) Sex differences in cerebral aneurysms and subarachnoid hemorrhage. Stroke 53(2):624–633. https://doi.org/10.1161/STROKEAHA.121.037147
Gathier CS, van den Bergh WM, van der Jagt M, Verweij BH, Dankbaar JW, Muller MC, Oldenbeuving AW, Rinkel GJE, Slooter AJC, Group HS (2018) Induced hypertension for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a randomized clinical trial. Stroke 49:76–83. https://doi.org/10.1161/STROKEAHA.117.017956
Germans MR, Jaja BNR, de Oliviera Manoel AL, Cohen AH, Macdonald RL (2018) Sex differences in delayed cerebral ischemia after subarachnoid hemorrhage. J Neurosurg 129:458–464. https://doi.org/10.3171/2017.3.JNS162808
Ghods AJ, Lopes D, Chen M (2012) Gender differences in cerebral aneurysm location. Front Neurol 3:78. https://doi.org/10.3389/fneur.2012.00078
Handa H, Hashimoto N, Nagata I, Hazama F (1983) Saccular cerebral aneurysms in rats: a newly developed animal model of the disease. Stroke 14:857–866. https://doi.org/10.1161/01.str.14.6.857
Hao Y, Liu J, Liu J, Yang N, Smith SC Jr, Huo Y, Fonarow GC, Ge J, Taubert KA, Morgan L, Zhou M, Xing Y, Ma CS, Han Y, Zhao D (2019) Sex differences in in-hospital management and outcomes of patients with acute coronary syndrome. Circulation 139:1776–1785. https://doi.org/10.1161/CIRCULATIONAHA.118.037655
Huttunen J, Kurki MI, von Und Zu, Fraunberg M, Koivisto T, Ronkainen A, Rinne J, Jaaskelainen JE, Kalviainen R, Immonen A (2015) Epilepsy after aneurysmal subarachnoid hemorrhage: a population-based, long-term follow-up study. Neurology 84:2229–2237. https://doi.org/10.1212/WNL.0000000000001643
Jung SY, Bae HJ, Park BJ, Yoon BW, Acute Brain Bleeding Analysis Study G (2010) Parity and risk of hemorrhagic strokes. Neurology 74:1424–1429. https://doi.org/10.1212/WNL.0b013e3181dc13a5
Lindekleiv H, Sandvei MS, Njolstad I, Lochen ML, Romundstad PR, Vatten L, Ingebrigtsen T, Vik A, Mathiesen EB (2011) Sex differences in risk factors for aneurysmal subarachnoid hemorrhage: a cohort study. Neurology 76:637–643. https://doi.org/10.1212/WNL.0b013e31820c30d3
Lindekleiv HM, Valen-Sendstad K, Morgan MK, Mardal KA, Faulder K, Magnus JH, Waterloo K, Romner B, Ingebrigtsen T (2010) Sex differences in intracranial arterial bifurcations. Gend Med 7:149–155. https://doi.org/10.1016/j.genm.2010.03.003
Lindgren A, Turner EB, Sillekens T, Meretoja A, Lee JM, Hemmen TM, Koivisto T, Alberts M, Lemmens R, Jaaskelainen JE, Vergouwen MDI, Rinkel GJE, Stroke Goal Group DFGCPDFL, Dr Foster Unit at Imperial College L (2019) Outcome after clipping and coiling for aneurysmal subarachnoid hemorrhage in clinical practice in Europe, USA, and Australia. Neurosurgery 84:1019–1027. https://doi.org/10.1093/neuros/nyy223
Muehlschlegel S (2018) Subarachnoid hemorrhage. Continuum (Minneap Minn) 24:1623–1657. https://doi.org/10.1212/CON.0000000000000679
Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ (2009) Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 8:635–642. https://doi.org/10.1016/S1474-4422(09)70126-7
Raine R, Goldfrad C, Rowan K, Black N (2002) Influence of patient gender on admission to intensive care. J Epidemiol Community Health 56:418–423. https://doi.org/10.1136/jech.56.6.418
Rinkel GJ, Djibuti M, Algra A, van Gijn J (1998) Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 29:251–256. https://doi.org/10.1161/01.str.29.1.251
Rosenlrn J, Eskesen V, Schmidt K (1993) Clinical features and outcome in females and males with ruptured intracranial saccular aneurysms. Br J Neurosurg 7:287–290. https://doi.org/10.3109/02688699309023811
Shufelt CL, Pacheco C, Tweet MS, Miller VM (2018) Sex-specific physiology and cardiovascular disease. Adv Exp Med Biol 1065:433–454. https://doi.org/10.1007/978-3-319-77932-4_27
Turan N, Heider RA, Zaharieva D, Ahmad FU, Barrow DL, Pradilla G (2016) Sex Differences in the formation of intracranial aneurysms and incidence and outcome of subarachnoid hemorrhage: review of experimental and human studies. Transl Stroke Res 7:12–19. https://doi.org/10.1007/s12975-015-0434-6
Vajda J (1992) Multiple intracranial aneurysms: a high risk condition. Acta Neurochir (Wien) 118:59–75. https://doi.org/10.1007/BF01400727
van Dijck J, Mostert CQB, Greeven APA, Kompanje EJO, Peul WC, de Ruiter GCW, Polinder S (2020) Functional outcome, in-hospital healthcare consumption and in-hospital costs for hospitalised traumatic brain injury patients: a Dutch prospective multicentre study. Acta Neurochir (Wien) 162:1607–1618. https://doi.org/10.1007/s00701-020-04384-9
Wang SS, Bogli SY, Nierobisch N, Wildbolz S, Keller E, Brandi G (2022) Sex-Related differences in patients’ characteristics, provided care, and outcomes following spontaneous intracerebral hemorrhage. Neurocrit Care 37(1):111–120. https://doi.org/10.1007/s12028-022-01453-y
Young AM, Karri SK, Ogilvy CS (2012) Exploring the use of estrogen & progesterone replacement therapy in subarachnoid hemorrhage. Curr Drug Saf 7:202–206. https://doi.org/10.2174/157488612803251261
Zacharia BE, Hickman ZL, Grobelny BT, DeRosa P, Kotchetkov I, Ducruet AF, Connolly ES Jr (2010) Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am 21:221–233. https://doi.org/10.1016/j.nec.2009.10.002
Funding
Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was performed in accordance with the ethical standards as laid down in the 2013 Declaration of Helsinki.
Consent to participate
Patients’ written agreement to have their data analyzed for research projects was obtained. In case of impossibility of the patient to give his/her agreement, a next of kin was asked to represent patient’s will.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurosurgical Intensive Care.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bögli, S.Y., Utebay, D., Smits, N. et al. Sex-related differences of invasive therapy in patients with aneurysmal subarachnoid hemorrhage. Acta Neurochir 164, 2899–2908 (2022). https://doi.org/10.1007/s00701-022-05345-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-022-05345-0