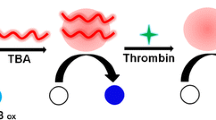
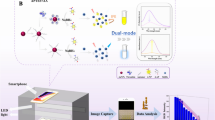
Abstract
Fibrinogen-modified gold nanoparticles (Fib-AuNPs) and 3,3′,5,5′-tetramethylbenzidine (TMB) substrate besides hydrogen peroxide (H2O2) were applied for assessment of the biomarker thrombin. Fib-AuNPs have catalytic active sites for the oxidation of TMB besides H2O2 and cause the color change of the substrate. Moreover, they can lead to the enhancement in the absorption wavelengths of 650 and 370 nm. By the addition of thrombin to Fib-AuNPs, fibrinogen turns into fibrin, and AuNPs are surrounded by fibrin. Therefore, their active catalytic sites for the oxidation of TMB besides H2O2 are covered by fibrin and cannot cause color change and absorption increase as before. The relationship between the average variations of the color intensity and changes in the absorption wavelengths at 650 and 350 nm with different concentrations of bovine thrombin added to Fib-AuNPs was studied. In such manner, three sensitive colorimetric approaches have been developed for the identification of bovine thrombin with the linear range of 20–120 pM and the limit of detection (LOD) of 17.54 pM for the average color intensity (G + B), the linear range of 20–120 pM and the LOD of 13.41 pM for the absorption peak at 650 nm, and the linear range of 40–140 pM with the LOD of 18.85 pM for absorption peak at 370 nm. The practical application of this biosensing platform was indicated through the successful determination of bovine thrombin in bovine serum. The satisfactory RSD ( < 10%) and recovery values (99.11–107.61%) confirmed the feasibility of the fabricated sensor.
Graphical abstract





Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Davie EW, Kulman JD (2006) An overview of the structure and function of thrombin. In: Seminars in thrombosis and hemostasis. Copyright© 2006 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New ….
Rawlings ND, Salvesen G (2013) Thrombin. In: Handbook of proteolytic enzymes, 3rd edn. Elsevier, p 2915–2932
Lin J-H et al (2019) Inhibition of catalytic activity of fibrinogen-stabilized gold nanoparticles via thrombin-induced inclusion of nanoparticle into fibrin: application for thrombin sensing with more than 104-fold selectivity. Spectrochim Acta Part A Mol Biomol Spectrosc 210:59–65
Kim H, An Z, Jang C-H (2018) Label-free optical detection of thrombin using a liquid crystal-based aptasensor. Microchem J 141:71–79
Zhang L, Li L (2016) Colorimetric thrombin assay using aptamer-functionalized gold nanoparticles acting as a peroxidase mimetic. Microchim Acta 183:485–490
Li L et al (2018) Target binding and DNA hybridization-induced gold nanoparticle aggregation for colorimetric detection of thrombin. Sens Actuators, B Chem 262:733–738
Chen C-K, Huang C-C, Chang H-T (2010) Label-free colorimetric detection of picomolar thrombin in blood plasma using a gold nanoparticle-based assay. Biosens Bioelectron 25(8):1922–1927
Hanson SR, Tucker EI, Latour RA (2020) Blood coagulation and blood–material interactions. In: Biomaterials Science: An introduction to materials in medicine, 4th edn. Elsevier, pp 801–812
Gonzalez E, Moore EE, Moore HB (2016) Fibrinogen. In: Trauma induced coagulopathy, Springer, Cham, pp 75–90
Park J et al (2019) Ultrasensitive detection of fibrinogen using erythrocyte membrane-draped electrochemical impedance biosensor. Sens Actuators, B Chem 293:296–303
Mosesson MW (2008) Structure and functions of fibrinogen and fibrin. Recent Adv Thromb Hemos 2008:3–26
Chen C-K et al (2011) Using self-assembled aptamers and fibrinogen-conjugated gold nanoparticles to detect DNA based on controlled thrombin activity. Biosens Bioelectron 26(8):3464–3468
Niu Y et al (2013) Turn-on colorimetric sensor for ultrasensitive detection of thrombin using fibrinogen–gold nanoparticle conjugate. Analyst 138(5):1475–1482
Chang C-C et al (2019) Gold nanoparticle-based colorimetric strategies for chemical and biological sensing applications. Nanomaterials 9(6):861
Wang H et al (2021) Recent advances in nanomaterials for colorimetric cancer detection. J Mater Chem B 9(4):921–938
Bigdeli A et al (2017) Nanoparticle-based optical sensor arrays. Nanoscale 9(43):16546–16563
Zeng J et al (2020) Anisotropic plasmonic nanostructures for colorimetric sensing. Nano Today 32:100855
Askim JR, Mahmoudi M, Suslick KS (2013) Optical sensor arrays for chemical sensing: the optoelectronic nose. Chem Soc Rev 42(22):8649–8682
Sun J et al (2020) Colorimetric sensor array based on gold nanoparticles: design principles and recent advances. TrAC, Trends Anal Chem 122:115754
Nie L et al (2014) Applications of gold nanoparticles in optical biosensors. J Biomed Nanotechnol 10(10):2700–2721
Wang H et al (2019) Noble metal nanoparticles growth-based colorimetric strategies: From monocolorimetric to multicolorimetric sensors. Coord Chem Rev 398:113003
Yazdian-Robati R et al (2021) Application of the catalytic activity of gold nanoparticles for development of optical aptasensors. Anal Biochem 629:114307
Huang X et al (2018) Smartphone-based analytical biosensors. Analyst 143(22):5339–5351
Dungchai W, Chailapakul O, Henry CS (2011) A low-cost, simple, and rapid fabrication method for paper-based microfluidics using wax screen-printing. Analyst 136(1):77–82
Shen L, Hagen JA, Papautsky I (2012) Point-of-care colorimetric detection with a smartphone. Lab Chip 12(21):4240–4243
McKeating KS et al (2013) An investigation into the simultaneous enzymatic and SERRS properties of silver nanoparticles. Analyst 138(21):6347–6353
Grabar KC et al (1995) Preparation and characterization of Au colloid monolayers. Anal Chem 67(4):735–743
Haiss W et al (2007) Determination of size and concentration of gold nanoparticles from UV− Vis spectra. Anal Chem 79(11):4215–4221
Swinehart DF (1962) The beer-lambert law. J Chem Educ 39(7):333
Rahman S (2016) Size and concentration analysis of gold nanoparticles with ultraviolet-visible spectroscopy. Undergrad J Math Model: One+ Two 7(1):2
Wolberg AS, Campbell RA (2008) Thrombin generation, fibrin clot formation and hemostasis. Transfus Apheres Sci 38(1):15–23
Zhang Z et al (2010) Magnetic nanoparticle-linked colorimetric aptasensor for the detection of thrombin. Sens Actuators, B Chem 147(2):428–433
Chen Y-Y et al (2011) Gold nanoparticle-based colorimetric assays for coagulation-related proteins and their inhibition reactions. Biosens Bioelectron 26(7):3160–3166
Liang G et al (2011) Magnetic relaxation switch and colorimetric detection of thrombin using aptamer-functionalized gold-coated iron oxide nanoparticles. Anal Chim Acta 689(2):243–249
Li J et al (2014) A non-aggregation colorimetric assay for thrombin based on catalytic properties of silver nanoparticles. Anal Chim Acta 807:120–125
Peng Y et al (2013) Aptamer-gold nanoparticle-based colorimetric assay for the sensitive detection of thrombin. Sens Actuators, B Chem 177:818–825
Chen Z et al (2014) A colorimetric aptamer biosensor based on cationic polymer and gold nanoparticles for the ultrasensitive detection of thrombin. Biosens Bioelectron 56:46–50
Chen Z et al (2016) Real colorimetric thrombin aptasensor by masking surfaces of catalytically active gold nanoparticles. ACS Appl Mater Interfaces 8(1):102–108
Liu M, Li J, Li B (2017) A colorimetric aptamer biosensor based on cationic polythiophene derivative as peroxidase mimetics for the ultrasensitive detection of thrombin. Talanta 175:224–228
Song HP et al (2018) Convenient colorimetric detection of thrombin via aptamer-mediated inhibition and restoration of the oxidase activity of nanoceria. J Nanosci Nanotechnol 18(9):6570–6574
Shen M, Wang Y, Kan X (2021) Dual-recognition colorimetric sensing of thrombin based on surface-imprinted aptamer–Fe 3 O 4. J Mater Chem B 9(20):4249–4256
Funding
This work was supported by the Biofuel and Renewable Energy Research Center, Department of Chemical Engineering, Babol Noshirvani University of Technology, Babol, Iran (grant number BNUT/370393/2023).
Author information
Authors and Affiliations
Contributions
MHA: conceptualization, methodology, validation, formal analysis, investigation, resources, writing—original draft, visualization, project administration. MR: conceptualization, methodology, resources, writing—review and editing, supervision, funding acquisition. HE: conceptualization, methodology, validation, investigation, writing—review and editing.
Corresponding author
Ethics declarations
Animal welfare
The study on laboratory animals for determination of Thrombin in this research was conducted at Babol University of Medical Sciences and was approved with the ethics ID of IR.MUBABOL.AEC.1401.026.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amani, M.H., Rahimnejad, M. & Ezoji, H. Smartphone-assisted quantitative colorimetric identification of thrombin based on peroxidase mimetic features of fibrinogen-gold nanozymes. Microchim Acta 191, 83 (2024). https://doi.org/10.1007/s00604-023-06173-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06173-4