Abstract
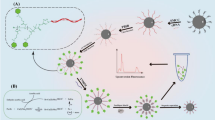
A simple fluorescence resonance energy transfer (FRET) sensing platform (termed as USP), comprised of upconversion nanoparticles (UCNPs) as the energy donor and Cy5 as the energy acceptor, has been synthesized for cathepsin B (CTSB) activity detection in vitro and in vivo. When Cy5-modified peptide substrate (peptide-Cy5) of CTSB is covalently linked on the surface of UCNPs, the FRET between the UCNPs (excitation: 980 nm; emission: 541 nm/655 nm) and Cy5 (excitation: 645 nm) leads to a reduction in the red upconversion luminescence (UCL) signal intensity of UCNPs. Cy5 can be liberated from UCNPs in the presence of CTSB through the cleavage of peptide-Cy5 by CTSB, leading to the recovery of the red UCL signal of UCNPs. Because the green UCL signal of UCNPs remains constant during the CTSB digestion, it can be considered as an internal reference. The findings demonstrate the ability of USP to detect CTSB with the linear detection ranges of 1 to 100 ng·mL−1 in buffer and 2 × 103 to 1 × 105 cells in 0.2 mL cell lysates. The limits of detection (LODs) are 0.30 ng·mL−1 in buffer and 887 cells in 0.2 mL of cell lysates (S/N = 3). The viability of USP to detect CTSB activity in tumor-bearing mice is has further been investigated using in vivo fluorescent imaging.
Graphical abstract






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Anderson MJ, Song Y, Fan H, Wright JG, Ren Z, Hua DH, Koehne JE, Meyyappan M, Li J (2020) Simultaneous, multiplex quantification of protease activities using a gold microelectrode array. Biosens Bioelectron 165:112330. https://doi.org/10.1016/j.bios.2020.112330
Dai Z, Cheng Q, Zhang Y (2020) Rational design of a humanized antibody inhibitor of cathepsin B. Biochemistry 59:1420–1427. https://doi.org/10.1021/acs.biochem.0c00046
Jin X, Yang H, Mao Z, Wang B (2021) Cathepsin B-responsive multifunctional peptide conjugated gold nanorods for mitochondrial targeting and precise photothermal cancer therapy. J Colloid Interface Sci 601:714–726. https://doi.org/10.1016/j.jcis.2021.05.135
Mijanović O, Branković A, Panin AN, Savchuk S, Timashev P, Ulasov I, Lesniak MS (2019) Cathepsin B: a sellsword of cancer progression. Cancer Lett 449:207–214. https://doi.org/10.1016/j.canlet.2019.02.035
Chen X, Ren X, Zhu Y, Fan Z, Zhang L, Liu Z, Dong L, Hai Z (2021) Cathepsin B-activated fluorescent and photoacoustic imaging of tumor. Anal Chem 93:9304–9308. https://doi.org/10.1021/acs.analchem.1c02145
Mitrović A, Završnik J, Mikhaylov G, Knez D, Pečar Fonović U, Matjan Štefin P, Butinar M, Gobec S, Turk B, Kos J (2022) Evaluation of novel cathepsin-X inhibitors in vitro and in vivo and their ability to improve cathepsin-B-directed antitumor therapy. Cell Mol Life Sci 79:34. https://doi.org/10.1007/s00018-021-04117-w
Shim MK, Moon Y, Yang S, Kim J, Cho H, Lim S, Yoon HY, Seong JK, Kim K (2020) Cancer-specific drug-drug nanoparticles of pro-apoptotic and cathepsin B-cleavable peptide-conjugated doxorubicin for drug-resistant cancer therapy. Biomaterials 261:120347. https://doi.org/10.1016/j.biomaterials.2020.120347
Wang S, Vigliarolo BG, Chowdhury MA, Nyarko JNK, Mousseau DD, Phenix CP (2019) Design and synthesis of fluorogenic substrate-based probes for detecting cathepsin B activity. Bioorg Chem 92:103194. https://doi.org/10.1016/j.bioorg.2019.103194
Adamo M, Sun G, Qiu D, Valente J, Lan W, Song H, Bolgar M, Katiyar A, Krishnamurthy G (2017) Drug-to-antibody determination for an antibody-drug-conjugate utilizing cathepsin B digestion coupled with reversed-phase high-pressure liquid chromatography analysis. J Chromatogr A 1481:44–52. https://doi.org/10.1016/j.chroma.2016.12.051
Kim CJ, Lee DI, Kim C, Lee K, Lee CH, Ahn IS (2014) Gold nanoparticles-based colorimetric assay for cathepsin B activity and the efficiency of its inhibitors. Anal Chem 86:3825–3833. https://doi.org/10.1021/ac4039064
Song Y, Fan H, Anderson MJ, Wright JG, Hua DH, Koehne J, Meyyappan M, Li J (2019) Electrochemical activity assay for protease analysis using carbon nanofiber nanoelectrode arrays. Anal Chem 91:3971–3979. https://doi.org/10.1021/acs.analchem.8b05189
Li CH, Lv WY, Yan Y, Yang FF, Zhen SJ, Huang CZ (2021) Nucleolin-targeted DNA nanotube for precise cancer therapy through Förster resonance energy transfer-indicated telomerase responsiveness. Anal Chem 93:3526–3534. https://doi.org/10.1021/acs.analchem.0c04917
Yuan Y, Zhang CJ, Gao M, Zhang R, Tang BZ, Liu B (2015) Specific light-up bioprobe with aggregation-induced emission and activatable photoactivity for the targeted and image-guided photodynamic ablation of cancer cells. Angew Chem Int Ed Engl 54:1780–1786. https://doi.org/10.1002/anie.201408476
Lin G, Jin D (2021) Responsive sensors of upconversion nanoparticles. ACS Sens 6:4272–4282. https://doi.org/10.1021/acssensors.1c02101
Xiang J, Zhou S, Lin J, Wen J, Xie Y, Yan B, Yan Q, Zhao Y, Shi F, Fan H (2021) Low-power near-infrared-responsive upconversion nanovectors. ACS Appl Mater Interfaces 13:7094–7101. https://doi.org/10.1021/acsami.0c21115
Yang N, Gong F, Cheng L (2022) Recent advances in upconversion nanoparticle-based nanocomposites for gas therapy. Chem Sci 13:1883–1898. https://doi.org/10.1039/d1sc04413c
Li H, Wang X, Ohulchanskyy TY, Chen G (2021) Lanthanide-doped near-infrared nanoparticles for biophotonics. Adv Mater 33:e2000678. https://doi.org/10.1002/adma.202000678
Li Y, Chen C, Liu F, Liu J (2022) Engineered lanthanide-doped upconversion nanoparticles for biosensing and bioimaging application. Microchim Acta 189:109. https://doi.org/10.1007/s00604-022-05180-1
Marturano V, Kozlowska J, Bajek A, Giamberini M, Ambrogi V, Cerruti P, Garcia-Valls R, Montornes JM, Tylkowski B (2019) Photo-triggered capsules based on lanthanide-doped upconverting nanoparticles for medical applications. Coord Chem Rev 398:7. https://doi.org/10.1016/j.ccr.2019.213013
Plunkett S, El Khatib M, Şencan İ, Porter JE, Kumar ATN, Collins JE, SakadŽić S, Vinogradov SA (2020) In vivo deep-tissue microscopy with UCNP/Janus-dendrimers as imaging probes: resolution at depth and feasibility of ratiometric sensing. Nanoscale 12:2657–2672. https://doi.org/10.1039/c9nr07778b
Sun C, Gradzielski M (2022) Advances in fluorescence sensing enabled by lanthanide-doped upconversion nanophosphors. Adv Colloid Interface Sci 300:102579. https://doi.org/10.1016/j.cis.2021.102579
Yi Z, Luo Z, Qin X, Chen Q, Liu X (2020) Lanthanide-activated nanoparticles: a toolbox for bioimaging, therapeutics, and neuromodulation. Acc Chem Res 53:2692–2704. https://doi.org/10.1021/acs.accounts.0c00513
Zhu X, Su Q, Feng W, Li F (2017) Anti-Stokes shift luminescent materials for bio-applications. Chem Soc Rev 46:1025–1039. https://doi.org/10.1039/c6cs00415f
Alonso-Cristobal P, Vilela P, El-Sagheer A, Lopez-Cabarcos E, Brown T, Muskens OL, Rubio-Retama J, Kanaras AG (2015) Highly sensitive DNA sensor based on upconversion nanoparticles and graphene oxide. ACS Appl Mater Interfaces 7:12422–12429. https://doi.org/10.1021/am507591u
Ding S, Wu W, Peng T, Pang W, Jiang P, Zhan Q, Qi S, Wei X, Gu B, Liu B (2021) Near-infrared light excited photodynamic anticancer therapy based on UCNP@AIEgen nanocomposite. Nanoscale Adv 3:2325–2333. https://doi.org/10.1039/d0na00985g
Liu L, Zhang H, Wang Z, Song D (2019) Peptide-functionalized upconversion nanoparticles-based FRET sensing platform for caspase-9 activity detection in vitro and in vivo. Biosens Bioelectron 141:111403. https://doi.org/10.1016/j.bios.2019.111403
Wang C, Li X, Zhang F (2016) Bioapplications and biotechnologies of upconversion nanoparticle-based nanosensors. Analyst 141:3601–3620. https://doi.org/10.1039/c6an00150e
Fang Y, Li Y, Li Y, He R, Zhang Y, Zhang X, Liu Y, Ju H (2021) In situ protease secretion visualization and metastatic lymph nodes imaging via a cell membrane-anchored upconversion nanoprobe. Anal Chem 93:7258–7265. https://doi.org/10.1021/acs.analchem.1c00469
Feng Y, Chen H, Ma L, Shao B, Zhao S, Wang Z, You H (2017) Surfactant-free aqueous synthesis of novel Ba2GdF7:Yb3+, Er3+@PEG upconversion nanoparticles for in vivo trimodality imaging. ACS Appl Mater Interfaces 9:15096–15102. https://doi.org/10.1021/acsami.7b03411
Tian R, Zhang H, Chen H, Liu G, Wang Z (2018) Uncovering the binding specificities of lectins with cells for precision colorectal cancer diagnosis based on multimodal imaging. Adv Sci 5:1800214. https://doi.org/10.1002/advs.201800214
Li X, Liu L, Fu Y, Chen H, Abualrejal MMA, Zhang H, Wang Z, Zhang H (2020) Peptide-enhanced tumor accumulation of upconversion nanoparticles for sensitive upconversion luminescence/magnetic resonance dual-mode bioimaging of colorectal tumors. Acta Biomater 104:167–175. https://doi.org/10.1016/j.actbio.2020.01.003
Yamini S, Gunaseelan M, Gangadharan A, Lopez SA, Martirosyan KS, Girigoswami A, Roy B, Manonmani J, Jayaraman S (2021) Upconversion, MRI imaging and optical trapping studies of silver nanoparticle decorated multifunctional NaGdF4:Yb,Er nanocomposite. Nanotechnology 33. https://doi.org/10.1088/1361-6528/ac37e4
Yamini S, Gunaseelan M, Kumar GA, Singh S, Dannangoda GC, Martirosyan KS, Sardar DK, Sivakumar S, Girigoswami A, Senthilselvan J (2020) NaGdF4:Yb, Er-Ag nanowire hybrid nanocomposite for multifunctional upconversion emission, optical imaging, MRI and CT imaging applications. Microchim Acta 187:317. https://doi.org/10.1007/s00604-020-04285-9
Li Y, Zhang X, Zhang Y, Zhang Y, He Y, Liu Y, Ju H (2020) Activatable photodynamic therapy with therapeutic effect prediction based on a self-correction upconversion nanoprobe. ACS Appl Mater Interfaces 12:19313–19323. https://doi.org/10.1021/acsami.0c03432
Song Y, Wright JG, Anderson MJ, Rajendran S, Ren Z, Hua DH, Koehne JE, Meyyappan M, Li J (2021) Quantitative detection of cathepsin B activity in neutral pH buffers using gold microelectrode arrays: toward direct multiplex analyses of extracellular proteases in human serum. ACS Sens 6:3621–3631. https://doi.org/10.1021/acssensors.1c01175
Liu L, Li XT, Zhang H, Chen HD, Abualrejal MMA, Song DQ, Wang ZX (2021) Six-in-one peptide functionalized upconversion@polydopamine nanoparticle-based ratiometric fluorescence sensing platform for real-time evaluating anticancer efficacy through monitoring caspase-3 activity. Sens Actuator B-Chem 333:8. https://doi.org/10.1016/j.snb.2021.129554
Chen HD, Zhang H, Wang ZX (2021) Development of small animals in vivo fluorescence-photothermal dual mode imaging system. Chem J Chin Univ.-Chin 42:725–730. https://doi.org/10.7503/cjcu20200640
Roth-Konforti ME, Bauer CR, Shabat D (2017) Unprecedented sensitivity in a probe for monitoring cathepsin B: chemiluminescence microscopy cell-imaging of a natively expressed enzyme. Angew Chem Int Ed Engl 56:15633–15638. https://doi.org/10.1002/anie.201709347
Wang Y, Li J, Feng L, Yu J, Zhang Y, Ye D, Chen HY (2016) Lysosome-targeting fluorogenic probe for cathepsin B imaging in living cells. Anal Chem 88:12403–12410. https://doi.org/10.1021/acs.analchem.6b03717
Funding
The authors would like to thank the National Natural Science Foundation of China (Grant No. 81871406 and 61901438) and the Achievement Transformation Guidance Fund Between the First Hospital of Jilin University and Changchun Institute of Applied Chemistry (Grant No. CGZHYD202012-009) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Zhang, M., Zhang, H. et al. Upconversion nanoparticle-based fluorescence resonance energy transfer sensing platform for the detection of cathepsin B activity in vitro and in vivo. Microchim Acta 190, 181 (2023). https://doi.org/10.1007/s00604-023-05771-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05771-6