Abstract
The development of an electrochemical procedure for the determination of 4-ethylguaiacol and its application to wine analysis is described. Modified screen-printed carbon electrodes (SPCEs) with fullerene C60 (C60) have been shown to be efficient in this kind of analysis. The developed activated C60/SPCEs (AC60/SPCEs) were adequate for the determination of 4-ethylguaicol, showing a linear range from 200 to 1000 µg/L, a reproducibility of 7.6% and a capability of detection (CCβ) value of 200 µg/L, under optimized conditions. The selectivity of the AC60/SPCE sensors was evaluated in the presence of possibly interfering compounds, and their practical applicability was demonstrated in the analysis of different wine samples obtaining recoveries ranging from 96 to 106%.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The occurrence of unpleasant odours in wine that negatively affect its quality may be related to the presence of certain molecules, including volatile phenols, such as 4-ethylguaiacol, associated to the advent of smoked aromas [1, 2]. Therefore, the development of analytical methods that allow the early detection of this compound is extremely important to avoid the rejection of a wine, which seriously affects the reputation and economy of the producing winery.
Most of the methods described for the quantification of 4-ethylguaicol in wine are based on the use of chromatographic techniques, being gas chromatography using mass spectrometric [3,4,5,6,7,8,9,10] and flame ionization [11,12,13,14,15] detection systems the most often selected technique in the analysis of this compound due to its volatility [3,4,5,6,7,8,9,10,11,12,13,14,15,16]. Wine is a complex matrix with many different constituents present at different concentration levels, considered low in the case of volatile phenols (between 1 and 2660 µg/L) [4]. Thus, the measurement of 4-ethylguaiacol in wine using gas chromatographic techniques frequently involves previous sample preparation steps based on different extraction procedures, including liquid–liquid extraction [3, 4], solid-phase extraction [10, 11, 14, 15] and stir bar sorptive extraction [8, 9]. Liquid chromatography has also been selected as an analytical technique in the determination of 4-ethylguaiacol in wine using diode array [17, 18] and fluorescence [17, 19] detectors, although the number of methods developed is considerably lower compared to gas chromatography. These chromatographic procedures also involve the use of preparative steps based on solvent and solid-phase extraction processes. The main advantages of the described chromatographic techniques are their high precision, selectivity and sensitivity. However, these techniques are associated with high-cost laboratory equipment and highly qualified personnel requirements, being difficult to adapt for in situ measurements, for example in a winery during the wine production process [16].
Electrochemical sensors may be an interesting alternative to chromatographic techniques in the determination of 4-ethylguaiacol since they can easily be oriented towards in situ analysis, being able to detect the cause of the contamination just at the moment it occurs with also high sensitivity and selectivity [20, 21]. Like this, 4-ethylguaicol has been analysed using different electrochemical techniques (Table 1) including screen-printed carbon electrodes (SPCEs) modified with TiO2 or SnO2 nanoparticles [22]. The joint determination of different phenols has also been approached using electrochemical sensors. Thus, the analysis of 4-ethylguaiacol in the presence of other phenols can be carried out using Nafion-modified boron-doped diamond electrodes in the presence of α-cyclodextrin [23] or by means of an array of different modified electrodes using artificial neural networks for quantitative analysis [20, 24, 25]. Despite the advantages of the described electrochemical methods, only one of these works involves the determination of 4-ethylguaicol in wine, but the detection limit reached (5.5 mg/L) above the normal content in wine samples [20]. Thus, the electrochemical analysis of this compound in complex samples, such as wine, requires the development of more sensitive and selective devices. In this way, highly selective sensors for the analysis of other volatile compounds in wine have been established by means of the modification of the working electrode surface with a molecularly imprinted polypyrrole polymer [26]. However, experiments carried out with this type of modification in this work, using 4-ethylguaicol as target molecule, did not lead to satisfactory results in its analysis in wine samples. On the other hand, the modification of the working electrode with nanomaterials has already proven to be suitable for the development of sensors for the analysis of similar compounds due to its capability to accept and donate electrons [27]. Among them, fullerene C60 (C60) has demonstrated to be an excellent material for electrode modification due to its high electroactive surface area and good conductivity. Moreover, C60-modified electrodes have shown a long stability and a wide potential window [28]. Therefore, disposable SPCEs have been modified with C60 for the development of sensitive and selective suitable sensors for the determination of 4-ethylguaiacol in wine. In addition, a selectivity improvement has been carried out in these analyses through a previous accumulation step of the analyte present in the gas phase set over the liquid sample [27].
Experimental
Reagents
Reagents used were analytical reagent grade chemicals, and all solutions were prepared using Milli-Q water (18.2 MΩ/cm; Millipore, Bedford, MA, USA). 4-Ethylguaicol was purchased from Alfa Aesar (98%; Haverhill, MA, USA); 0.1 M phosphate (KH2PO4) buffer solutions (Fluka, Munich, Germany) containing 0.1 M potassium chloride (Merck, Darmstadt, Germany) were used as supporting electrolyte for the electrochemical measurements; 1 M phosphoric acid (85%; Panreac, Barcelona, Spain) was used to adjust the pH of the buffer solutions to the adequate value; C60 solutions (99.9%; Acros Organics, Geel, Belgium), used in the C60/SPCE generation, were prepared using dichloromethane, purchased from Panreac (Barcelona, Spain), as solvent; 1.0 M KOH solutions (Carlo Erba, Val de Reuil, France) were used in the reduction of these modified electrodes; and 4-ethylphenol (97%; Alfa Aesar, Haverhill, MA, USA), 4-vinylphenol (10 wt% solution in propylene glycol; SAFC, St. Louis, MO, USA) and p-coumaric acid (> 98%; Sigma-Aldrich, Steinheim, Germany) were analysed as possible interferences.
Preparation of AC 60 /GCEs
The modification of the SPCE surface (DRP-C11L; Metrohm DropSens, Oviedo, Spain) with a well-coated layer of C60 (i.e. C60/SPCE) was performed according to a previously described procedure [27]. Briefly, 40 µL of 0.1 mg/mL solution of C60, prepared in dichloromethane, was deposited on the SPCE surface and allowed to dry at room temperature. This C60 film formed on the electrode surface was next partially reduced in 1.0 M KOH by cyclic voltammetry in the potential range from 0.0 to − 1.5 V vs. Ag/AgCl, at a scan rate of 10 mV/s, becoming conductive due to the formation of K3C60 salt [29]. Thus, an activated C60/SPCE (AC60/SPCE) was obtained in this way. The formation of the AC60 layer on the SPCE can be seen in the scanning electron microscope (SEM) images shown in Fig. 1.
Electrochemical measurements with AC 60 /GCEs
Electrochemical measurements were carried out by means of differential pulse voltammetry (DPV) by means of a PalmSens4 potentiostat (PalmSens BV, Houten, The Netherlands), using a previous accumulation step of the analyte on the electrode. First, the AC60/SPCE was introduced into the top of a sealed cell containing 1 mL of supporting electrolyte solution (pH 2.3), except for the optimization process, and the corresponding analyte concentration. The solution was stirred during 360 s at 70 °C. After this incubation time, in which the 4-ethyguaicol present in the gas phase over this solution was accumulated on the working electrode, differential pulse voltammograms were performed in a drop of 100 µL of supporting electrolyte solution deposited on the AC60/GCE.
Results
The electrochemical oxidation of 4-ethylguaicol to the corresponding quinone has been described as a process which involves the previous formation of an unstable phenoxy radical in a one-electron and one-proton step [23, 30]. This oxidation response gives rise to an analytical signal suitable for the sensitive determination of this compound using conventional electrodes. However, it may show a lack of selectivity when the analysis of 4-ethylguaicol is carried out on complex samples. The modification of the working electrode with a molecularly imprinted polypyrrole polymer [26] and with C60 [27] has therefore been studied to avoid this possible loss of selectivity. Better results were achieved in the case of the nanomaterial, so they are the only ones that will be shown below. In this case, SPCEs were selected considering their better properties to those of conventional electrodes, including their low cost that allows the simple production of a large number of disposable devices with numerous possibilities of modification and, in addition, their ease of adaptation to small portable instrument systems [31].
The oxidation of 4-ethylguaicol to its corresponding quinone may be observed using an AC60/SPCE by means of DPV in a 100 µL droplet dropped of supporting electrolyte directly onto the three-electrode system, after a previous accumulation of the analyte on the electrode surface in the gas phase, to increase the selectivity of the analytical method. This oxidation signal was influenced by different parameters, including temperature and incubation time applied during the accumulation step, as well as pH of the supporting electrolyte solution used for accumulation and DPV measurements. Thus, in order to ensure that the analytical measurements were carried out under the best possible conditions, an optimization stage of these variables was first performed. Experimental designs were used as a tool for optimization which allow to explore a wide experimental range with a reduced number of experiments. Moreover, they are more effective than the “one-at-time” experiments being able to detect interactions between the different factors that could lead to wrong decisions [32]. A 23 central composite experimental design was then performed, taking the following values as high, low and central levels for each of the factors to be optimized, being the oxidation intensity obtained for a 0.98 mg/L 4-ethylguaiacol solution the response to be optimized (Table 2).
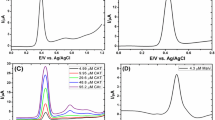
Seventeen experiments were consequently performed with different combinations of these values, including three replicates in the central point to evaluate the residual error (Table 3). From the analysis of the results obtained for the different experiments, the following optimum values for the variables were found: pH, 2.3; incubation time, 12 min; and incubation temperature, 70 °C, using Statgraphics program [33]. Under these optimized conditions, an oxidation peak was observed at a potential of + 0.48 V vs. Ag/AgCl, which increased with the increasing analyte concentration (Fig. 2a, b) and being the sensitivity of the AC60/SPCEs much higher than that obtained using bare SPCEs (Fig. 2c). Thus, the modification of the electrode surface by an activated film of C60 notably improves the reactivity of a SPCE.
a DPV curves and b experimental points and calibration plot obtained under optimized experimental conditions in the 4-ethylguaicol concentration ranging from 200 to 1000 µg/L using an AC60/SPCE. c DPV curves obtained for a 2 mg/L 4-ethylguaicol solution using different electrodes (phosphate; pH, 2.3; incubation time, 12 min; incubation temperature, 70 °C; pulse potential, + 0.2 V; step potential, + 0.01 V; pulse time, 0.02 s; and scan rate, 50 mV/s)
An incubation time of 12 min implies spending excessively time in the construction of each calibration set, which is difficult to adapt for real-time analysis. Thus, a deep study of the influence of incubation time on the sensitivity value of the method was carried out. Different calibration sets were constructed in the concentration range between 200 and 1000 µg/L, using different incubation times ranging from 3 to 6 min. As it can be seen in Fig. 3a, the sensitivity value found for 6 min was similar to that obtained for 12 min. Thus, this value was selected for next experiments, obtaining as well defined DPV signals for the quantification of 4-ethylguaicol (Fig. 3b).
a Experimental points and calibration plots obtained using an AC60/SPCE for different incubation times ((1) 3 min [blue], (2) 4 min [yellow], (3) 5 min [green] and (4) 6 min [red]). b DPV curves obtained in the 4-ethylguaicol concentration ranging from 200 to 1000 µg/L using an AC60/SPCE (phosphate; pH, 2.3; incubation time, 6 min; incubation temperature, 70 °C; pulse potential, + 0.2 V; step potential, + 0.01 V; pulse time, 0.02 s; and scan rate, 50 mV/s)
Finally, the developed procedure based on AC60/SPCEs was validated under the optimized conditions for the 4-ethylguaicol determination by means of the estimation of its precision, capability of detection, decision limit and trueness. Hence, different calibration plots were built using ordinary linear regressions in the concentration ranging from 200 to 1000 µg/L. Outlier points, characterized by a studentized residual higher than 2.5, in absolute value, were rejected with the aim to achieve a perfect evaluation of the different calibration parameters [33]. The precision of the method was then assessed by studying the reproducibility obtained for the slopes of three different validated calibration sets, in order to evaluate the dispersion referred to a range of concentrations instead of just a single concentration value. The value of relative standard deviation (RSD) obtained was 7.6%, showing a high degree of precision.
Capability of detection (CCβ) and decision limit (CCα) were calculated using DETARCHI program [34], according to the ISO 11843 approach, based on a linear regression model [35]. A value of 58.7 µg/L was obtained for CCα, for a probability of false positive (α) and negative (β) of 0.05. The value found for CCβ was inferior than that of the concentration used in the first calibration point, so 200 µg/L was taken as the capability of detection of the method.
Interference analysis
The selectivity towards 4-ethylguaicol of the developed AC60/SPCEs was also analysed by studying the possible interference caused by the presence of other compounds with a similar structure, including 4-ethylphenol, 4-vinylphenol and p-coumaric acid. Thus, different solutions of the possible interferent with concentrations between 500 and 1400 µg/L were analysed, keeping the concentration of 4-ethylguaicol at a constant value of 700 µg/L in all of them. Some grade of interference in the determination of 4-ethylguaicol was found for 4-ethylphenol at concentrations higher than 800 µg/L, due to the high overlap of the oxidation peaks of both phenols (Fig. 4a). 4-Vinylphenol and p-coumaric acid showed no influence on the oxidation signal of 4-ethylguaicol even at high concentration levels (1400 µg/L) as it can be seen in Fig. 4b and c.
DPV curves obtained for solutions containing 700 µg/L of 4-ethylguaicol and different concentrations of a 4-ethylphenol, b 4-vinylphenol and c p-coumaric acid using an AC60/SPCE (phosphate; pH, 2.3; incubation time, 6 min; incubation temperature, 70 °C; pulse potential, + 0.2 V; step potential, + 0.01 V; pulse time, 0.02 s; and scan rate, 50 mV/s)
Wine sample analysis
The developed AC60/SPCE sensors were also validated in terms of trueness by means of their application to the analysis of 4-ethylguaicol in different wine samples. Two commercial samples of red wine (Tempranillo variety) and two of white wine (Airen and Verdejo varieties) were studied, not finding the presence of 4-ethylguaicol in any of them. Thus, an analysis of spiked wine samples was performed by means of standard addition method, obtaining results far from the concentration with which the samples were enriched. Hence, an important matrix effect was observed, which was avoided using a liquid–liquid extraction procedure for each standard: 2 mL of diethyl ether was added to 5 mL of spiked wine sample and ultrasonicated for 5 min. Then, 100 µL of the ether layer was transfer to a vial containing 900 µL of pH 2.3 phosphate buffer solution for the accumulation step. The DPV analysis of the extracted phases according to this standard addition procedure gave rise to results shown in Table 4. As it can be seen, there is significant agreement of the results obtained by the described procedure and the real content of 4-ethylguaicol of the spiked wine samples, obtaining good recovery percentages between 96 and 106% which confirm the potential of the developed sensors for practical applications.
Conclusions
This work describes an electrochemical method that combines the important advantages of SPCEs, related to its low cost and possibility of adaptation to in situ analysis, with the use of nanomaterials such as C60 for its modification, which provides improved sensitivity in the analysis of 4-ethylguaiacol. In addition, the prior accumulation of the analyte present in the gas phase on the electrode surface manages to improve selectivity, allowing its analysis in the range of concentrations normally present in wine. The developed sensors are then presented as an interesting alternative to the more complex and expensive classical techniques used by wine producers for the analysis of 4-ethylguaiacol.
References
Kheir J, Salameh D, Strehaiano P, Brandam C, Lteif R (2013) Impact of volatile phenols and their precursors on wine quality and control measures of Brettanomyces/Dekkera yeasts. Eur Food Res Technol 237:655–671. https://doi.org/10.1007/s00217-013-2036-4
Larcher R, Nicolini G, Puecher C, Bertoldi D, Moser S, Favaro G (2007) Determination of volatile phenols in wine using high-performance liquid chromatography with a coulometric array detector. Anal Chim Acta 582:55–60. https://doi.org/10.1016/j.aca.2006.08.056
Milheiro J, Filipe-Ribeiro L, Cosme F, Nunes FM (2017) A simple, cheap and reliable method for control of 4-ethylphenol and 4-ethylguaiacol in red wines. Screening of fining agents for reducing volatile phenols levels in red wines. J Chromatogr B Anal Technol Biomed Life Sci 1041–1042:183–190. https://doi.org/10.1016/j.jchromb.2016.10.036
Pollnitz AP, Pardon KH, Sefton MA (2000) Quantitative analysis of 4-ethylphenol and 4-ethylguaiacol in red wine. J Chromatogr A 874:101–109. https://doi.org/10.1016/S0021-9673(00)00086-8
Carpinteiro I, Abuín B, Rodríguez I, Ramil M, Cela R (2012) Mixed-mode solid-phase extraction followed by dispersive liquid-liquid microextraction for the sensitive determination of ethylphenols in red wines. J Chromatogr A 1229:79–85. https://doi.org/10.1016/j.chroma.2012.01.044
Carpinteiro I, Abuín B, Rodríguez I, Ramil M, Cela R (2010) Sorptive extraction with in-sample acetylation for gas chromatography-mass spectrometry determination of ethylphenol species in wine samples. J Chromatogr A 1217:7208–7214. https://doi.org/10.1016/j.chroma.2010.09.036
Fariña L, Boido E, Carrau F, Dellacassa E (2007) Determination of volatile phenols in red wines by dispersive liquid-liquid microextraction and gas chromatography-mass spectrometry detection. J Chromatogr A 1157:46–50. https://doi.org/10.1016/j.chroma.2007.05.006
Marín J, Zalacain A, De Miguel C, Alonso GL, Salinas MR (2005) Stir bar sorptive extraction for the determination of volatile compounds in oak-aged wines. J Chromatogr A 1098:1–6. https://doi.org/10.1016/j.chroma.2005.07.126
Díez J, Domínguez C, Guillén DA, Veas R, Barroso CG (2004) Optimisation of stir bar sorptive extraction for the analysis of volatile phenols in wines. J Chromatogr A 1025:263–267. https://doi.org/10.1016/j.chroma.2003.10.073
López R, Aznar M, Cacho J, Ferreira V (2002) Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J Chromatogr A 966:167–177. https://doi.org/10.1016/S0021-9673(02)00696-9
Domínguez C, Guillén DA, Barroso CG (2002) Determination of volatile phenols in fino sherry wines. Anal Chim Acta 458:95–102. https://doi.org/10.1016/S0003-2670(01)01581-1
Sun X, Luo X, Ma T, You Y, Huang W, Zhan J (2017) Detection method optimization, dynamic changes during alcoholic fermentation and content analysis of “Brett Character” compounds 4-ethylphenol (4-EP) and 4-ethylguaiacol (4-EG) in Chinese red wines. Food Anal Methods 10:1616–1629. https://doi.org/10.1007/s12161-016-0719-8
Monje MC, Privat C, Gastine V, Nepveu F (2002) Determination of ethylphenol compounds in wine by headspace solid-phase microextraction in conjunction with gas chromatography and flame ionization detection. Anal Chim Acta 458:111–117. https://doi.org/10.1016/S0003-2670(01)01528-8
Castro Mejías R, Natera Marín R, García Moreno MDV, García Barroso C (2003) Optimisation of headspace solid-phase microextraction for the analysis of volatile phenols in wine. J Chromatogr A 995:11–20. https://doi.org/10.1016/S0021-9673(03)00541-7
Martorell N, Martí MP, Mestres M, Busto O, Guasch J (2002) Determination of 4-ethylguaiacol and 4-ethylphenol in red wines using headspace-solid-phase microextraction-gas chromatography. J Chromatogr A 975:349–354. https://doi.org/10.1016/S0021-9673(02)01277-3
Milheiro J, Filipe-Ribeiro L, Vilela A, Cosme F, Nunes FM (2019) 4-Ethylphenol, 4-ethylguaiacol and 4-ethylcatechol in red wines: microbial formation, prevention, remediation and overview of analytical approaches. Crit Rev Food Sci Nutr 59:1367–1391. https://doi.org/10.1080/10408398.2017.1408563
Caboni P, Sarais G, Cabras M, Angioni A (2007) Determination of 4-ethylphenol and 4-ethylguaiacol in wines by LC-MS-MS and HPLC-DAD-fluorescence. J Agric Food Chem 55:7288–7293. https://doi.org/10.1021/jf071156m
Garcia D, Gomez-Caballero A, Guerreiro A, Goicolea MA, Barrio RJ (2015) Molecularly imprinted polymers as a tool for the study of the 4-ethylphenol metabolic pathway in red wines. J Chromatogr A 1410:164–172. https://doi.org/10.1016/j.chroma.2015.07.103
Nikfardjam MP, May B, Tschiersch C (2009) 4-Ethylphenol and 4-ethylguaiacol contents in bottled wines from the German “Württemberg” region. Eur Food Res Technol 230:333–341. https://doi.org/10.1007/s00217-009-1174-1
González-Calabuig A, del Valle M (2018) Voltammetric electronic tongue to identify Brett character in wines. On-site quantification of its ethylphenol metabolites. Talanta 179:70–74. https://doi.org/10.1016/j.talanta.2017.10.041
Garcia-Mutio D, Gomez-Caballero A, Guerreiro A, Piletsky S, Goicolea MA, Barrio RJ (2016) Solid-phase synthesis of imprinted nanoparticles grafted on gold substrates for voltammetric sensing of 4-ethylphenol. Sensors Actuators B Chem 236:839–848. https://doi.org/10.1016/j.snb.2016.02.018
Fang Y, Umasankar Y, Ramasamy RP (2014) Electrochemical detection of p-ethylguaiacol, a fungi infected fruit volatile using metal oxide nanoparticles. Analyst 139:3804–3810. https://doi.org/10.1039/c4an00384e
Hayes PE, Glennon JD, Buzid A, Luong JHT (2020) Simultaneous electroanalysis of guaiacol and its analogs based on their differential complexation with α-cyclodextrin on Nafion modified boron-doped diamond electrode. Electroanalysis 32:119–127. https://doi.org/10.1002/elan.201900403
Kalinke C, de Oliveira PR, Bonet San Emeterio M, González-Calabuig A, del Valle M, SalvioMangrich A, Humberto Marcolino Junior L, Bergamini MF (2019) Voltammetric electronic tongue based on carbon paste electrodes modified with biochar for phenolic compounds stripping detection. Electroanalysis 31:2238–2245. https://doi.org/10.1002/elan.201900072
Herrera-Chacon A, González-Calabuig A, Campos I, del Valle M (2018) Bioelectronic tongue using MIP sensors for the resolution of volatile phenolic compounds. Sensors Actuators B Chem 258:665–671. https://doi.org/10.1016/j.snb.2017.11.136
Domínguez-Renedo O, Navarro-Cuñado AM, Arnáiz-Lozano V, Alonso-Lomillo MA (2020) Molecularly imprinted polypyrrole based electrochemical sensor for selective determination of 4-ethylphenol. Talanta 207:120351. https://doi.org/10.1016/j.talanta.2019.120351
Portugal-Gómez P, Asunción Alonso-Lomillo M, Domínguez-Renedo O (2022) 4-ethyphenol detection in wine by fullerene modified screen-printed carbon electrodes. Microchem J 180:107599. https://doi.org/10.1016/j.microc.2022.107599
Goyal RN, Gupta VK, Chatterjee S (2009) Fullerene-C60-modified edge plane pyrolytic graphite electrode for the determination of dexamethasone in pharmaceutical formulations and human biological fluids. Biosens Bioelectron 24:1649–1654. https://doi.org/10.1016/j.bios.2008.08.024
Szücs A, Tölgyesi M, Szücs E, Nagy JB, Novák M (1997) On the mixed doping of fullerene films. J Electroanal Chem 429:27–35. https://doi.org/10.1016/S0022-0728(97)00136-8
Enache TA, Oliveira-Brett AM (2011) Phenol and para-substituted phenols electrochemical oxidation pathways. J Electroanal Chem 655:9–16. https://doi.org/10.1016/j.jelechem.2011.02.022
Domínguez-Renedo O, Alonso-Lomillo MA, Arcos-Martínez MJ (2007) Recent developments in the field of screen-printed electrodes and their related applications. Talanta 73:202–219. https://doi.org/10.1016/j.talanta.2007.03.050
Morgan E (1991) Chemometrics: Experimental Design (Analytical Chemistry by Open Learning). Wiley, New York
Statgraphics Centurion 19 (2021) Statgraphics Technologies Inc
Sarabia L, Ortiz MC (1994) DETARCHI: a program for detection limits with specified assurance probabilities and characteristic curves of detection. TrAC - Trends Anal Chem 13:1–6. https://doi.org/10.1016/0165-9936(94)85052-6
ISO 11843-2 (2000) Capability of detection (part I: terms and definitions,1997. Part 2: methodology in the linear calibration case). Geneva, Switzerland
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was supported by the Agencia Estatal de Investigación/Ministerio de Ciencia e Innovación under the project code PID2020-117095RB-I00.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally in the conceptualisation, data analysis, and writing of original draft and revision. Funding acquisition was performed by M. A. Alonso-Lomillo and O. Domínguez-Renedo. Data collection was performed by P. Portugal-Gómez and A. M. Navarro-Cuñado. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Portugal-Gómez, P., Navarro-Cuñado, A.M., Alonso-Lomillo, M.A. et al. Electrochemical sensors for the determination of 4-ethylguaiacol in wine. Microchim Acta 190, 141 (2023). https://doi.org/10.1007/s00604-023-05729-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05729-8