Abstract
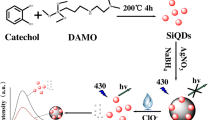
Inorganic pyrophosphate anions (PPi) play a key role in various biological processes and act as an essential indicator for physiological function evaluation and disease diagnosis. However, there is still a lack of available approaches for straightforward, robust, and convenient PPi detection. Herein, we design an on-off-on fluorescent switching nanoprobe employing Fe3+-mediated fluorescent sulfur quantum dots (SQDs) for highly robust detection of PPi. The bovine serum protein (BSA)-capped SQDs with fine water dispersibility and good optical stability are synthesized by an H2O2-assisted chemical etching reaction. Specifically, Fe3+ can strongly induce the aggregation of the SQDs into relatively larger sizes, resulting in aggregation-induced fluorescence quenching behavior. PPi can selectively bind with Fe3+ via emulative coordination and in preventing the aggregation of SQDs this is accompanied by recovery of fluorescence. The physicochemical properties of aggregated and disaggregated SQDs have been systematically investigated. Aggregation and disaggregation of the SQDs and the corresponding quenching and recovery of fluorescence occurs and guarantees the high-contrast sensing performance of the SQD system in complex and challenging aquatic environments. Our designed on-off-on nanoswitch holds great potential for the design of elemental quantum dot-based biosensors for the highly robust detection of analytes in the near future.
Graphical abstract








Similar content being viewed by others
References
Doherty M, Belcher C, Regan M, Jones A, Ledingham J (1996) Association between synovial fluid levels of inorganic pyrophosphate and short term radiographic outcome of knee osteoarthritis. Ann Rheum Dis 55:432–436. https://doi.org/10.1136/ard.55.7.432
Lee S, Yuen KK, Jolliffe KA, Yoon J (2015) Fluorescent and colorimetric chemosensors for pyrophosphate. Chem Soc Rev 44:1749–1762. https://doi.org/10.1039/c4cs00353e
Terkeltaub RA (2001) Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol 281:C1–C11. https://doi.org/10.1152/ajpcell.2001.281.1.c1
Smith SA, Choi SH, Davis-Harrison R, Huyck J, Boettcher J, Rienstra CM, Morrissey JH (2010) Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood 116:4353–4359. https://doi.org/10.1182/blood-2010-01-266791
Correll DL (1998) The role of phosphorus in the eutrophication of receiving waters: a review. J Environ Qual 27:261–266. https://doi.org/10.2134/jeq1998.00472425002700020004x
Luo L, Chen Y, Zhang L, Li Y, Li H, Zhang H, Tian Y (2017) SERS assay for pyrophosphate based on its competitive binding to Cu (II) ion on silver nanoparticles modified with cysteine and rhodamine 6G. Microchim Acta 184:595–601. https://doi.org/10.1007/s00604-016-2044-8
Tanikawa H, Ogawa R, Okuma K, Harato K, Niki Y, Kobayashi S, Nagura T (2018) Detection of calcium pyrophosphate dihydrate crystals in knee meniscus by dual-energy computed tomography. J Orthop Surg 13:1–6. https://doi.org/10.1186/s13018-018-0787-0
Sivasankaran U, Reinke L, Anand SK, Malecka K, Kumar KG, Radecka H, Kubik S, Radecki S (2020) Ultrasensitive electrochemical sensing of phosphate in water mediated by a dipicolylamine-zinc (II) complex. Sens Actuators B Chem 321:128474. https://doi.org/10.1016/j.snb.2020.128474
Qi F, Han Y, Ye Z, Liu H, Wei L, Xiao L (2018) Color-coded single-particle pyrophosphate assay with dark-field optical microscopy. Anal Chem 90:11146–11153. https://doi.org/10.1021/acs.analchem.8b03211
Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T (2008) Quantum dots versus organic dyes as fluorescent labels. Nat Methods 5:763–775. https://doi.org/10.1038/nmeth.1248
Zhang Q, Deng S, Liu J, Zhong X, He J, Chen X, Feng B, Chen Y, Ostrikov K (2019) Cancer-targeting graphene quantum dots: fluorescence quantum yields, stability, and cell selectivity. Adv Funct Mater 29:1805860. https://doi.org/10.1002/adfm.201805860
Chinnathambi S, Chen S, Ganesan S, Hanagata N (2014) Silicon quantum dots for biological applications. Adv Healthcare Mater 3:10–29. https://doi.org/10.1002/adhm.201300157
Devi P, Saini S, Kim KH (2019) The advanced role of carbon quantum dots in nanomedical applications. Biosens Bioelectron 141:111158. https://doi.org/10.1016/j.bios.2019.02.059
Arshad F, Sk MP (2020) Luminescent sulfur quantum dots for colorimetric discrimination of multiple metal ions. ACS Appl Nano Mater 3:3044–3049. https://doi.org/10.1021/acsanm.0c00394
Song Y, Tan J, Wang G, Gao P, Lei J, Zhou L (2020) Oxygen accelerated scalable synthesis of highly fluorescent sulfur quantum dots. Chem Sci 11:772–777. https://doi.org/10.1039/c9sc05019a
Wang H, Wang Z, Xiong Y, Kershaw SV, Li T, Wang Y, Zhai Y, Rogach AL (2019) Hydrogen peroxide assisted synthesis of highly luminescent sulfur quantum dots. Angew Chem 131:7114–7118. https://doi.org/10.1002/anie.201902344
Arshad F, Sk MP, Maurya SK, Siddique HR (2021) Mechanochemical synthesis of sulfur quantum dots for cellular imaging. ACS Appl Nano Mater. 4:3339–3344. https://doi.org/10.1021/acsanm.1c00509
Zhang C, Zhang P, Ji X, Wang H, Kuang H, Cao W, Pan M, Shi Y, Wang Z (2019) Ultrasonication-promoted synthesis of luminescent sulfur nano-dots for cellular imaging applications. Chem Commun 55:13004–13007. https://doi.org/10.1039/c9cc06586e
Pal A, Arshad F, Sk MP (2020) Emergence of sulfur quantum dots: unfolding their synthesis, properties, and applications. Adv Colloid Interface Sci 285:102274. https://doi.org/10.1016/j.cis.2020.102274
Kaur J, Singh PK (2022) Nanomaterial based advancement in the inorganic pyrophosphate detection methods in the last decade: a review. Trends Anal. Chem 146:116483 https://www.sciencedirect.com/science/article/abs/pii/S016599362100306X
Tan J, Deng P, Wang L, Bi X, Zhou Y, Wang Y, Zhang P (2022) Signal-on and selective detection of anti-tumor adjunctive drug zoledronic acid using fluorescent sulfur quantum dots. Dyes Pigm 205:110520. https://doi.org/10.1016/j.dyepig.2022.110520
Gao P, Wang G, Zhou L (2020) Luminescent sulfur quantum dots: synthesis, properties and potential applications. Chem Photo Chem 4:5235–5244. https://doi.org/10.1002/cptc.202000158
Wang S, Bao X, Gao B, Li M (2019) A novel sulfur quantum dot for the detection of cobalt ions and norfloxacin as a fluorescent “switch”. Dalton Trans 48:8288–8296. https://doi.org/10.1039/c9dt01186b
Shen L, Wang H, Liu S, Bai Z, Zhang S, Zhang X, Zhang C (2018) Assembling of sulfur quantum dots in fission of sublimed sulfur. J Am Chem Soc 140:7878–7884. https://doi.org/10.1021/jacs.8b02792
Sun YP, Zhou B, Lin Y, Wang W, Fernando KS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG, Yang H, Kose ME, Chen B, Veca LM, Xie SY (2006) Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc 128:7756–7757. https://doi.org/10.1021/ja062677d
Mullamuri B, Bhagavathula SD, Kasturi KC, Reddy V (2017) Facile synthesis of bovine serum albumin conjugated low-dimensional ZnS nanocrystals. Int J Biol Macromol. 101:729–773. https://doi.org/10.1016/j.ijbiomac.2017.03.164
Yang L, Xing R, Shen Q, Jiang K, Ye F, Wang J, Ren Q (2006) Fabrication of protein-conjugated silver sulfide nanorods in the bovine serum albumin solution. J. Phys. Chem. B 110:10534–10539. https://doi.org/10.1021/jp055603h
Liu S, Li Y, Li L (2017) Enhanced stability and mechanical strength of sodium alginate composite films. Carbohydr. Polym 16:62–70 https://www.sciencedirect.com/science/article/abs/pii/S0144861716314254
Riahi Z, Priyadarshi R, Rhim JW, Lotfali E, Bagheri R, Pircheraghi G (2022) Alginate-based multifunctional films incorporated with sulfur quantum dots for active packaging applications. Colloid Surface B. 215:112519 https://www.sciencedirect.com/science/article/abs/pii/S0927776522002028
Schlabach MR, Bates GW (1975) The synergistic binding of anions and Fe3+ by transferrin. Implications for the interlocking sites hypothesis. J Biol Chem 250:2182–2188. https://doi.org/10.1016/S0021-9258(19)41699-2
Gao PX, Huang ZY, Tan JS, Lv GS, Zhou L (2022) Efficient conversion of elemental sulfur to robust ultrabright fluorescent sulfur quantum dots using sulfur-ethylenediamine precursor. ACS Sustainable Chem. Eng 10:4634–4641 https://pubs.acs.org/doi/abs/10.1021/acssuschemeng.2c00036
Park JH, Kumar N, Park DH, Yusupov M, Neyts EC, Verlackt C, Bogaerts A, Kang MH, Uhm HS, Choi EH (2015) A comparative study for the inactivation of multidrug resistance bacteria using dielectric barrier discharge and nanosecond pulsed plasma. Sci Rep 5:13849. https://doi.org/10.1038/srep13849
Wu M, Chen Y, Lin H, Zhao L, Shen L, Li R, Xu Y, Hong H, He Y (2020) Membrane fouling caused by biological foams in a submerged membrane bioreactor: mechanism insights. Water Res. 181:115932 https://www.sciencedirect.com/science/article/abs/pii/S0043135420304693
Mei Q, Jiang C, Guan G, Zhang K, Liu B, Liu R, Zhang Z (2012) Fluorescent graphene oxide logic gates for discrimination of iron (3+) and iron (2+) in living cells by imaging. Chem Commun 48:7468–7470. https://doi.org/10.1039/c2cc31992f
Deng HH, Wang FF, Shi XQ, Peng HP, Liu AL, Xia XH, Chen W (2016) Water-soluble gold nanoclusters prepared by protein-ligand interaction as fluorescent probe for real-time assay of pyrophosphatase activity. Biosens Bioelectron 83:1–8. https://doi.org/10.1016/j.bios.2016.04.031
Funding
This work was supported by the National Natural Science Foundation of China (no. 21775014 and 22204012), Chongqing Innovation Research Group Project (no. CXQT21015), Natural Science Foundation of Chongqing (no. CSTB2022NSCQ-MSX0466), and Startup Founds of Chongqing Normal University (no. 22XLB010).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
Appendix A. Supplementary data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, X., Cai, Q., Zhang, J. et al. Protein-directed synthesis of fluorescent sulfur quantum dots for highly robust detection of pyrophosphate. Microchim Acta 190, 104 (2023). https://doi.org/10.1007/s00604-023-05686-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05686-2