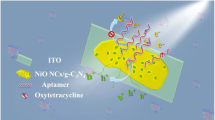
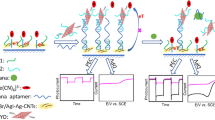
Abstract
Nanosheets of anatase TiO2 and CdS quantum dots modified with thioglycolic acid (TGA-CdS QDs) were prepared and hierarchically modified on the indium tin oxides (ITO) electrodes. The heterojunction structure is formed to improve the light capture ability and carrier migration, significantly enhancing the sensitivity of photoelectrochemical (PEC) biosensors. Specific DNA sequences labeled with TGA-CdS QDs were placed on the electrodes to prepare a biosensor for the detection of chloramphenicol with ultrahigh selectivity. In addition, the heterojunction structure and the principle of photocurrent signal amplification on the electrode are described in detail. Under the optimal conditions, the photoelectrochemical biosensors showed good reproducibility and stability for chloramphenicol with a linear response in the range 10–10,000 pM and a limit of detection (LOD) of 0.23 pM. Due to the specific recognition of base pairs, the sensor has excellent anti-interference ability in practical applications. An effective method was developed for the accurate detection of antibiotics with far reaching prospects.
Graphical Abstract






Similar content being viewed by others
References
Zhang B, Liu Z, Yang H, Yang X (2011) Methodological study on magnetic affinity immunoassay for detecting chloramphenicol. Chin J Cell Mol Immunol 27:585–589. https://doi.org/10.13423/j.cnki.cjcmi.006034
Sun T, Pan H, Mei Y, Zhang P, Zeng D, Liu X, Song S et al (2019) Electrochemical sensor sensitive detection of chloramphenicol based on ionic-liquid-assisted synthesis of de-layered molybdenum disulfide/graphene oxide nanocomposites. J Appl Electrochem 49:261–270. https://doi.org/10.1007/s10800-018-1271-6
Yu MT, Xia T, Bai WC, Ji JY, Wang H, Huang YQ et al (2020) Handheld aptasensor for sandwiched detection of chloramphenicol. Chem Res Chin Univ 36:291–295. https://doi.org/10.1007/s40242-020-9076-7
Zhu Y, Li X, Xu Y, W, L, Yu A, Lai G et al (2021) Intertwined carbon nanotubes and Ag nanowires constructed by simple solution blending as sensitive and stable chloramphenicol sensors. Sensors 21:1220. https://doi.org/10.3390/s21041220
Ben Y, Fu C, Hu M, Liu L, Wong M, Zheng C (2019) Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: a review. Environ Res 169:483–493. https://doi.org/10.1016/j.envres.2018.11.040
Zhong Y, Chen C, Liu S, Lu C, Liu D, Pan Y et al (2021) A new magnetic adsorbent of eggshell-zeolitic imidazolate framework for highly efficient removal of norfloxacin. Dalton Trans 50:18016–18026. https://doi.org/10.1039/d1dt03020e
Amiripour F, Ghasemi S, Azizi SN (2021) Design of turn-on luminescent sensor based on nanostructured molecularly imprinted polymer-coated zirconium metal-organic framework for selective detection of chloramphenicol residues in milk and honey. Food Chem 347:129034. https://doi.org/10.1016/j.foodchem.2021.129034
Navada KK, Kulal A (2019) Enzymatic degradation of chloramphenicol by laccase from Trametes hirsuta and comparison among mediators. Int Biodeterior Biodegrad 138:63–69. https://doi.org/10.1016/j.ibiod.2018.12.012
Veach BT (2020) Determination of chloramphenicol and nitrofuran metabolites in cobia, croaker, and shrimp using microwave-assisted derivatization, automated SPE, and LC-MS/MS—results from a US Food and Drug Administration Level Three Inter-Laboratory Study. J AOAC Int 103:1043–1051. https://doi.org/10.1093/jaoacint/qsaa019
Shi X, Song S, Sun A, Li D, Wu A, Zhang D (2010) Determination of chloramphenicol residues in foods by ELISA and LC-MS/MS coupled with molecularly imprinted solid phase extraction. Anal Lett 43:2798–2807. https://doi.org/10.1080/00032711003763616
Crofts TS, Sontha P, King AO, Wang B, Biddy BA, Zanolli et al (2019) Discovery and characterization of a nitroreductase capable of conferring bacterial resistance to chloramphenicol. Cell Chem Biol 26:559–570. https://doi.org/10.1016/j.chembiol.2019.01.007
Amelin VG, Volkova NM, Repin NA, Nikeshina TB (2015) Simultaneous determination of residual amounts of amphenicols in food by HPLC with UV-detection. J Anal Chem 70:1282–1287. https://doi.org/10.1134/S1061934815100020
Ali I, Aboul-Enein HY, Gupta VK, Singh P, Negi U (2009) Analyses of chloramphenicol in biological samples by HPLC. Anal Lett 42:1368–1381. https://doi.org/10.1080/00032710902954482
Li S, Ma X, Pang C, Wang M, Yin G, Xu, Z et al (2021) Novel chloramphenicol sensor based on aggregation-induced electrochemiluminescence and nanozyme amplification. Biosens Bioelectron 176:112944. https://doi.org/10.1016/j.bios.2020.112944
Dong B, Li H, Sun J, Mari GM, Ai J, Han D et al (2020) Homogeneous fluorescent immunoassay for the simultaneous detection of chloramphenicol and amantadine via the duplex FRET between carbon dots and WS2 nanosheets. Food Chem 327:127107. https://doi.org/10.1016/j.foodchem.2020.127107
Li W, Chen N, Huang Z, Zeng X, Zhu Y (2019) Switchable hydrophilicity dispersive solvent-based liquid-liquid microextraction coupling to high-performance liquid chromatography for the determination of amphenicols in food products. Food Anal Meth 12:517–525. https://doi.org/10.1007/s12161-018-1382-z
Shen C, Liu S, Li X, Yang M (2019) Electrochemical detection of circulating tumor cells based on DNA generated electrochemical current and rolling circle amplification. Anal Chem 91:11614–11619. https://doi.org/10.1021/acs.analchem.9b01897
Li X, Shen C, Yang M, Rasooly A (2018) Polycytosine DNA electric-current-generated immunosensor for electrochemical detection of human epidermal growth factor receptor2 (HER2). Anal Chem 90:4764–4769. https://doi.org/10.1021/acs.analchem.8b00023
Shen C, Zeng K, Luo J, Li X, Yang M, Rasooly A (2017) Self-assembled DNA generated electric current biosensor for HER2 analysis. Anal Chem 89:10264–10269. https://doi.org/10.1021/acs.analchem.7b01747
Bai X, Gao W, Zhou C, Zhao D, Zhang Y, Jia N (2022) Photoelectrochemical determination of diclofenac using oriented single-crystalline TiO2 nanoarray modified with molecularly imprinted polypyrrole. Microchim Acta 189:90. https://doi.org/10.1007/s00604-022-05206-8
Wang Q, Guo H, Wei Y, Xue R, Ma B, Yang W (2020) An ultrahighly sensitive electrochemical biosensor for cytochrome C with surface molecular imprinting based on hybrid nanomaterials. Int J Electrochem Sci 15:990–1004. https://doi.org/10.20964/2020.01.25
Wu ZW, Xie XC, Guo HR, Xia H, Huang KJ (2020) A highly sensitive electrochemical biosensor for protein based on a tetrahedral DNA probe, N- and P-co-doped graphene, and rolling circle amplification. Anal Bioanal Chem 412:915–922. https://doi.org/10.1007/s00216-019-02314-y
Amiri NS, Milani-Hosseini M-R (2019) Fabrication of an eco-friendly ratiometric fluorescence sensor-modified mesoporous-structured epitope-imprinted polymer for highly selective and sensitive determination of cytochrome c in biological samples. Anal Methods 11:5919–5928. https://doi.org/10.1039/c8ay02773k
Cakiroglu B, Ozacar M (2018) A self-powered photoelectrochemical glucose biosensor based on supercapacitor Co3O4-CNT hybrid on TiO2. Biosens Bioelectron 119:34–41. https://doi.org/10.1016/j.bios.2018.07.049
Dong S, Yan J, Zhou S, Zhou Q (2022) Mycotoxins detection based on electrochemical approaches. Electroanalysis 34:132–147. https://doi.org/10.1002/elan.202100349
Liu XP, Chen JS, Mao C, Niu HL, Song JM, Jin BK (2018) A label-free photoelectrochemical biosensor for urokinase-type plasminogen activator detection based on a g-C3N4/CdS nanocomposite. Anal Chim Acta 1025:99–107. https://doi.org/10.1016/j.aca.2018.04.051
Xia LY, Li MJ, Wang HJ, Yuan R, Chai YQ (2019) A novel “signal on” photoelectrochemical strategy based on dual functional hemin for microRNA assay. Chem Commun 55:9721–9724. https://doi.org/10.1039/c9cc04899e
Roxby DN, Rivy H, Gong C, Gong X, Yuan Z, Chang GE et al (2020) Microalgae living sensor for metal ion detection with nanocavity-enhanced photoelectrochemistry. Biosens Bioelectron 165:112420. https://doi.org/10.1016/j.bios.2020.112420
Forzato C, Vida V, Berti F (2020) Biosensors and sensing systems for rapid analysis of phenolic compounds from plants: a comprehensive review. Biosensors-Basel 10:105. https://doi.org/10.3390/bios10090105
Huixian S, Tianyong Z, Hongliang W, Meng H (2011) Photocatalytic conversion of naphthalene to alpha-naphthol using nanometer-sized TiO2. Chin J Catal 32:46–50. https://doi.org/10.1016/S1872-2067(10)60158-1
Mao J, Yao JN, Wang LN, Liu WS (2008) Easily prepared high-quantum-yield CdS quantum dots in water using hyperbranched polyethylenimine as modifier. J Colloid Interface Sci 319:353–356. https://doi.org/10.1016/j.jcis.2007.10.027
Yao J, Gou X (2016) An investigation of preparation, properties, characterization and the mechanism of zinc blende CdTe/CdS core/shell quantum dots for sensitive and selective detection of trace mercury. J Mater Chem C 4:9856–9863. https://doi.org/10.1039/c6tc02878k
Hao Q, Wang L, Niu S, Ding C, Luo X (2020) Ratiometric electrogenerated chemiluminescence sensor based on a designed anti-fouling peptide for the detection of carcinoembryonic antigen. Anal Chim Acta 1136:134–140. https://doi.org/10.1016/j.aca.2020.09.033
Ma X, Ma Y, Ejeromedoghene O, Kandawa-Schulz M, Song W, Wang Y (2022) Photoelectrochemical detection of microRNAs based on target-triggered self-assembly of energy band position-matched CdS QDs and C3N4 nanosheets. Microchim Acta 189:65. https://doi.org/10.1007/s00604-022-05168-x
Yao J, Li Y, Xie M, Yang Q, Liu T (2020) The electrochemical behaviors and kinetics of AuNPs/N, S-GQDs composite electrode: A novel label-free amplified BPA aptasensor with extreme sensitivity and selectivity. J Mol Liq 320:114384. https://doi.org/10.1016/j.molliq.2020.114384
Cao K, Chen MM, Chang FY, Cheng YY, Tian LJ, Li F et al (2020) The biosynthesis of cadmium selenide quantum dots by Rhodotorula mucilaginosa PA-1 for photocatalysis. Biochem Eng J 156:107497. https://doi.org/10.1016/j.bej.2020.107497
Meng L, Xiao K, Zhang X, Du C, Chen J (2020) A novel signal-off photoelectrochemical biosensor for M.SssI MTase activity assay based on GQDs@ZIF-8 polyhedra as signal quencher. Biosens Bioelectron 150:111861. https://doi.org/10.1016/j.bios.2019.111861
Gao X, Niu S, Ge J, Luan Q, Jie G (2020) 3D DNA nanosphere-based photoelectrochemical biosensor combined with multiple enzyme-free amplification for ultrasensitive detection of cancer biomarkers. Biosens Bioelectron 147:111778. https://doi.org/10.1016/j.bios.2019.111778
Li J, Yuan H, Li J, Zhang W, Liu Y, Liu N et al (2021) The significant role of the chemically bonded interfaces in BiVO4/ZnO heterostructures for photoelectrochemical water splitting. Appl Catal B-Environ 285:119833. https://doi.org/10.1016/j.apcatb.2020.119833
Dey T, Ghorai A, Das S, Ray SK (2022) Solvent-engineered performance improvement of graphene quantum dot sensitized solar cells with nitrogen functionalized GQD photosensitizers. Sol Energy 236:17–25. https://doi.org/10.1016/j.solener.2022.02.048
Zhu Y, Xu Z, Gao J, Ji W, Zhang J (2020) An antibody-aptamer sandwich cathodic photoelectrochemical biosensor for the detection of progesterone. Biosens Bioelectron 160:112210. https://doi.org/10.1016/j.bios.2020.112210
Cheng W, Zheng Z, Yang J, Chen M, Yao Q, Chen Y et al (2019) The visible light-driven and self-powered photoelectrochemical biosensor for organophosphate pesticides detection based on nitrogen doped carbon quantum dots for the signal amplification. Electrochim Acta 296:627–636. https://doi.org/10.1016/j.electacta.2018.11.086
Chang J, Lv W, Wu J, Li H, Li F (2021) Simultaneous photoelectrochemical detection of dual microRNAs by capturing CdS quantum dots and methylene blue based on target-initiated strand displaced amplification. Chin Chem Lett 32:775–778. https://doi.org/10.1016/j.cclet.2020.05.041
Xiao K, Meng L, Du C, Zhang Q, Yu Q, Zhang X et al (2021) A label-free photoelectrochemical biosensor with near-zero-background noise for protein kinase A activity assay based on porous ZrO2/CdS octahedra. Sens Actuator B-Chem 328:129096. https://doi.org/10.1016/j.snb.2020.129096
Yao J, Wang L, Zhou H, Xie Z, Zeng X, Liu C (2022) Cuprous oxide coated silver/graphitic carbon nitride/cadmium sulfide nanocomposite heterostructure: specific recognition of carcinoembryonic antigen through sandwich-type mechanism. J Colloid Interface Sci 616:858–870. https://doi.org/10.1016/j.jcis.2021.11.103
Chen X, Wu W, Zhang Q, Wang C, Fan Y, Wu H et al (2022) Z-scheme Bi2O3/CuBi2O4 heterojunction enabled sensitive photoelectrochemical detection of aflatoxin B1 for health care, the environment, and food. Biosens Bioelectron 214:114523. https://doi.org/10.1016/j.bios.2022.114523
Cui L, Shen J, Li CC, Cui PP, Luo X, Wang X et al (2021) Construction of a dye-sensitized and gold plasmon-enhanced cathodic photoelectrochemical biosensor for methyltransferase activity assay. Anal Chem 93:10310–10316. https://doi.org/10.1021/acs.analchem.1c01797
Yang J, Fu S, Luo F, Guo L, Qiu B, Lin Z (2021) Homogeneous photoelectrochemical biosensor for microRNA based on target-responsive hydrogel coupled with exonuclease III and nicking endonuclease Nb.BbvCI assistant cascaded amplification strategy. Microchim Acta 188:267. https://doi.org/10.1007/s00604-021-04935-6
Tu YP, Wang HH, Long D, Yuan R, Chai YQ (2022) Highly sensitive photoelectrochemical biosensor based on Au nanoparticles sensitized zinc selenide quantum dots for DNA detection. Sens Actuator B-Chem 357:131255. https://doi.org/10.1016/j.snb.2021.131255
Shan J, Li R, Yan K, Zhu Y, Zhang J (2016) In situ anodic stripping of Cd(II) from CdS quantum dots for electrochemical sensing of ciprofloxacin. Sens Actuator B-Chem 237:75–80. https://doi.org/10.1016/j.snb.2016.06.066
Joo J, Ye Y, Kim D, Lee J, Jeon S (2013) Magnetically recoverable hybrid TiO2 nanocrystal clusters with enhanced photocatalytic activity. Mater Lett 93:141–144. https://doi.org/10.1016/j.matlet.2012.10.067
Noipa T, Martwiset S, Butwong N, Tuntulani T, Ngeontae W (2011) Enhancement of the fluorescence quenching efficiency of DPPH center dot on colloidal nanocrystalline quantum dots in aqueous micelles. J Fluoresc 21:1941–1949. https://doi.org/10.1007/s10895-011-0893-4
Liu S, Wang H, Cheng Z, Liu H (2016) Hexametaphosphate-capped quantum dots as fluorescent probes for detection of calcium ion and fluoride. Sens Actuator B-Chem 232:306–312. https://doi.org/10.1016/j.snb.2016.03.077
Wang X, Liu Y, Ding B, Li H, Zhu X, Xia M et al (2018) Influence of the addition of nano-TiO2 and ZnO on the sensing performance of micro-ZnSnO3 ethanol sensors under UV illumination. Sens Actuator B-Chem 276:211–221. https://doi.org/10.1016/j.snb.2018.08.114
Smirnov MS (2021) Temperature features of non-radiative energy transfer in hybrid associates of CdS/TGA quantum dots with methylene blue molecules. J Nanopart Res 23:59. https://doi.org/10.1007/s11051-020-05065-5
Kathalingam A, Valanarasu S, Ahamad T, Alshehri SM, Kim H-S (2021) Spray pressure variation effect on the properties of CdS thin films for photodetector applications. Ceram Int 47:7608–7616. https://doi.org/10.1016/j.ceramint.2020.11.100
Chang H, Lv J, Zhang H, Zhang B, Wei W, Qiao Y (2017) Photoresponsive colorimetric immunoassay based on chitosan modified AgI/TiO2 heterojunction for highly sensitive chloramphenicol detection. Biosens Bioelectron 87:579–586. https://doi.org/10.1016/j.bios.2016.09.002
Qin X, Wang Q, Geng L, Shu X, Wang Y (2019) A “signal-on” photoelectrochemical aptasensor based on graphene quantum dots-sensitized TiO2 nanotube arrays for sensitive detection of chloramphenicol. Talanta 197:28–35. https://doi.org/10.1016/j.talanta.2018.12.103
Tu C, Dai Y, Zhang Y, Wang W, Wu L (2020) A simple fluorescent strategy based on triple-helix molecular switch for sensitive detection of chloramphenicol. Spectroc Acta Pt A-Molec Biomolec Spectr 224:117415. https://doi.org/10.1016/j.saa.2019.117415
Yu HL, Zhou YW, Liu LQ, Zhang MY, Wang JB, Chen XY et al (2021) Simple development of Kelvin probe using a pA meter and its application to study the photocatalytic activities of Ag/TiO2 and Ag2O/TiO2 coated polyester fabrics. Appl Surf Sci 535:147653. https://doi.org/10.1016/j.apsusc.2020.147653
Wu S, Li X, Tian Y, Lin Y, Hu YH (2021) Excellent photocatalytic degradation of tetracycline over black anatase-TiO2 under visible light. Chem Eng J 406:126747. https://doi.org/10.1016/j.cej.2020.126747
Maver K, Arcon I, Fanetti M, Emin S, Valant M, Stangar UL (2021) Improved photocatalytic activity of anatase-rutile nanocomposites induced by low-temperature sol-gel Sn-modification of TiO2. Catal Today 361:124–129. https://doi.org/10.1016/j.cattod.2020.01.045
Ming N, Tao S, Yang W, Chen Q, Sun R, Wang C et al (2018) Mode-locked Er-doped fiber laser based on PbS/CdS core/shell quantum dots as saturable absorber. Opt Express 26:9017–9026. https://doi.org/10.1364/OE.26.009017
Xu Y, Liu X, Zheng Y, Li C, Yeung KWK, Cui Z et al (2021) Ag3PO4 decorated black urchin-like defective TiO2 for rapid and long-term bacteria-killing under visible light. Bioact Mater 6:1575–1587. https://doi.org/10.1016/j.bioactmat.2020.11.013
Funding
This work was supported by the National Natural Science Foundation of China (No. 51802273), Sichuan Science and Technology Program (No. 19YYJC2882), Key Project of Southwest Petroleum University Open Experiments (No. 2020KSZ04030), Key Project of Southwest Petroleum University Open Experiments (2021KSZ04034), Ordinary Project of Southwest Petroleum University Open Experiments (No. 2021KSP04018), and Ordinary Project of Southwest Petroleum University Open Experiments (No. 2021KSP04019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, J., Zeng, X. Photoelectrochemical biosensor based on DNA aptamers and dual nano-semiconductor heterojunctions for accurate and selective sensing of chloramphenicol. Microchim Acta 190, 18 (2023). https://doi.org/10.1007/s00604-022-05573-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05573-2