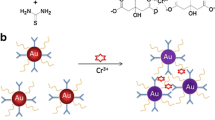
Abstract
Highly negatively charged gold nanoparticles (AuNPs) are shown to have strong simulated oxidase activity and effectively boosted the oxidation of enzyme substrate 3,3′,5,5′-tetramethylbenzidine (TMB) by hexavalent chromium ion Cr(VI), resulting in the formation of oxidation product with blue color. Based on this, a facile colorimetric assay was developed to detect Cr(VI) at a range 0.008~0.156 mg/L with r = 0.996. The detection limit was estimated to be 0.52 μg/L. In addition, the colorimetric assay showed high selectivity against 28 other interfering ions. It was performed at room temperature and required about half an hour including the preparation of AuNPs. The assay was successfully applied to the determination of Cr(VI) in spiked water samples, and recoveries in the range 95.00–105.40% were obtained. This work paves a way for design of high performance sensor based on highly active nanozymes and also provides an extremely practical analytical tool for the monitoring of Cr(VI) in the environment.
Graphical abstract







Similar content being viewed by others
References
Edition F (2011) Guidelines for drinking-water quality. WHO Chron 38:104–108
Hao C, Du P, Chen J, Hu SH, Li SQ, Liu HL (2010) Separation and preconcentration system based on ultrasonic probe-assisted ionic liquid dispersive liquid-liquid microextraction for determination trace amount of chromium(VI) by electrothermal atomic absorption spectrometry. Talanta 81(1–2):176–179
Zhan MH, Yu HM, Li LH, Nguyen DT, Chen W (2019) Detection of hexavalent chromium by copper sulfide nanocomposites. Anal Chem 91(3):2058–2065
Unceta N, Astorkia M, Abrego Z, Gómez-Caballero A, Goicolea MA, Barrio RJ (2016) A novel strategy for Cr(III) and Cr(VI) analysis in dietary supplements by speciated isotope dilution mass spectrometry. Talanta 154:255–262
Miyake Y, Tokumura M, Iwazaki Y, Wang Q, Amagai T, Horii Y, Otsuka H, Tanikawa N, Kobayashi T, Oguchi M (2017) Determination of hexavalent chromium concentration in industrial waste incinerator stack gas by using a modified ion chromatography with post-column derivatization method. J Chromatogr A 1502:24–29
Mädler S, Todd A, Skip Kingston HM, Pamuku M, Sun FR, Tat C, Tooley RJ, Switzer TA, Furdui VI (2016) Ultra-trace level speciated isotope dilution measurement of Cr(VI) using ion chromatography tandem mass spectrometry in environmental waters. Talanta 156–157:104–111
Motaghedifarda MH, Pourmortazavib SM, Mirsadeghic S (2020) Selective and sensitive detection of Cr(VI) pollution in waste water via polyaniline/sulfated zirconium dioxide/multi walled carbon nanotubes nanocomposite based electrochemical sensor. Sensors Actuators B Chem 327:128882
Prabhakaran DC, Ramamurthy PC, Sivry Y, Subramanian S (2020) Electrochemical detection of Cr(VI) and Cr(III) ions present in aqueous solutions using bio-modified carbon paste electrode: a voltammetric study. Int J Environ An Ch 21:1–21
Bu XF, Zhang ZY, Zhang LX, Peng L, Wu JW, Zhang HQ, Tian Y (2018) Highly sensitive SERS determination of chromium(VI) in water based on carbimazole functionalized alginate-protected silver nanoparticles. Sensors Actuators B Chem 273:1519–1524
Qian JN, Huang N, Lu QY, Wen C, Xia JB (2020) A novel D-A-D-typed rod-like fluorescent material for efficient Fe(III) and Cr(VI) detection: synthesis, structure and properties. Sensors Actuators B Chem 320:128377
Yang YZ, Chen XM, Wang YY, Wu M, Ma YN, Yang XD (2020) A novel fluorescent test papers based on carbon dots for selective and sensitive detection of Cr (VI). Front Chem 8:1130
Bilgic A, Cimen A (2020) A highly sensitive and selective ON-OFF fluorescent sensor based on functionalized magnetite nanoparticles for detection of Cr(VI) metal ions in the aqueous medium. J Mol Liq 312:113398
Kim D, Choi E, Lee C, Choi Y, Kim H, Yu T, Piao Y (2019) Highly sensitive and selective visual detection of Cr(VI) ions based on etching of silver-coated gold nanorods. Nano Converg 6(1):34
Zhang T, Zhang ST, Liu J, Li J, Lu XQ (2020) Visual chemosensor for hexavalent chromium via a controlled strategy for signal amplification in water. Anal Chem 92(4):3426–3433
He SZ, Lin X, Liang H, Xiao FB, Li FF, Liu C, Fan PF, Yang SY, Liu Y (2019) Colorimetric detection of Cr(VI) using silver nanoparticles functionalized with PVP. Anal Methods 11:5819–5825
Ghayyem S, Swaidan A, Barras A, Dolci M, Faridbod F, Szunerits S, Boukherroub R (2021) Colorimetric detection of chromium (VI) ion using poly(N-phenylglycine) nanoparticles acting as a peroxidase mimetic catalyst. Talanta 226:122082
Zhang XH, Liu W, Li XM, Zhang Z, Shan DL, Xia H, Zhang ST, Lu XQ (2018) Ultrahigh selective colorimetric quantification of chromium(VI) ions based on gold amalgam catalyst oxidoreductase-like activity in water. Anal Chem 90(24):14309–14315
Xue QS, Li X, Peng YX, Liu P, Peng HB, Niu XH (2020) Polyethylenimine-stabilized silver nanoclusters act as an oxidoreductase mimic for colorimetric determination of chromium(VI). Microchim Acta 187(5):263
Alula MT, Madingwane ML (2020) Colorimetric quantification of chromium (VI) ions based on oxidoreductase-like activity of Fe3O4. Sensors Actuators B Chem 324:128726
Wu XY, Xu YB, Dong YJ, Jiang X, Zhu NN (2013) Colorimetric determination of hexavalent chromium with ascorbic acid capped silver nanoparticles. Anal Methods 5:560–565
Zhuang YT, Chen S, Jiang R, Yu YL, Wang JH (2019) Ultrasensitive colorimetric chromium chemosensor based on dye color switching under the Cr(VI)-stimulated Au NPs catalytic activity. Anal Chem 91(8):5346–5353
Li HX, Rothberg L (2004) Colorimetric detection of DNA sequences based on electrostatic interactions with unmodifed gold nanoparticles. P Natl Acad Sci USA 101(39):14036–14039
Qi YY, Song DD, Chen YT (2021) Colorimetric oligonucleotide-based sensor for ultra-low Hg2+ in contaminated environmental medium: convenience, sensitivity and mechanism. Sci Total Environ 766:142579
Jv Y, Li BX, Cao R (2010) Positively-charged gold nanoparticles as peroxidase mimic and their application in hydrogen peroxide and glucose detection. Chem Commun 46(42):8017–8019
Chen HY, Qiu QM, Sharif S, Ying SN, Wang YX, Ying YB (2018) Solution-phase synthesis of platinum nanoparticle-decorated metal-organic framework hybrid nanomaterials as biomimetic nanoenzymes for biosensing applications. ACS Appl Mater Inter 10(28):24108–24115
Peng YG, Yu XP, Yin WQ, Dong WF, Peng Y, Wang T (2019) Colorimetric assay using mesoporous Fe-doped graphitic carbon nitride as a peroxidase mimetic for the determination of hydrogen peroxide and glucose. ACS Applied Bio Materials 3(1):59–67
Cui YS, Lai X, Liang B, Liang Y, Sun ST, Wang LG (2020) Polyethyleneimine-stabilized platinum nanoparticles as peroxidase mimic for colorimetric detection of glucose. ACS Omega 5(12):6800–6808
Qi YY, Chen YT, He JH, Gao X (2020) Highly sensitive and simple colorimetric assay of hydrogen peroxide and glucose in human serum via the smart synergistic catalytic mechanism. Spectrochim Acta A 234:118233
Zhou XB, Wang MK, Chen JY, Xie XL, Su XG (2020) Peroxidase-like activity of Fe-N-C single-atom nanozyme based colorimetric detection of galactose. Anal Chim Acta 1128:72–79
Nghia NN, Huy BT, Lee Y (2019) Colorimetric detection of chromium(VI) using graphene oxide nanoparticles acting as a peroxidase mimetic catalyst and 8-hydroxyquinoline as an inhibitor. Microchim Acta 186(1):36
Wang Y, Liang RP, Qiu JD (2020) Nanoceria-templated metal organic frameworks with oxidase-mimicking activity boosted by hexavalent chromium. Anal Chem 92(2):2339–2346
Mao Y, Gao SJ, Yao LL, Wang L, Qu H, Wu Y, Chen Y, Zheng L (2021) Single-atom nanozyme enabled fast and highly sensitive colorimetric detection of Cr(VI). J Hazard Mater 408:124898
Qi YY, Li BX (2011) A sensitive, label-free, aptamer-based biosensor using a gold nanoparticle-initiated chemiluminescence system. Chem Eur J 17(5):1642–1648
Zhang ZF, Cui H, Lai CZ, Liu LJ (2005) Gold nanoparticle-catalyzed luminol chemiluminescence and its analytical applications. Anal Chem 77(10):3324–3329
Qi YY, Xiu FR, Yu GD, Huang LL, Li BX (2017) Simple and rapid chemiluminescence aptasensor for Hg2+ in contaminated samples: a new signal amplification mechanism. Biosens Bioelectron 87:439–446
Sui CX, Liu YF, Zhang WH, Li PA, Zhang D (2014) CdTe-CdSe nanocrystals capped with dimethylaminoethanethiol as ultrasensitive fluorescent probes for chromium(VI). Microchim Acta 181:347–353
Huang K, Yang H, Zhou ZG, Yu MX, Li FY, Gao X, Yi T, Huang CH (2008) Multisignal chemosensor for Cr3+ and its application in bioimaging. Org Lett 10:2557–2560
Wu Y, Zheng J, Li Z, Zhao Y, Zhang Y (2009) A novel reagentless amperometric immunosensor based on gold nanoparticles/TMB/Nafion-modified electrode. Biosens Bioelectron 24(5):1389–1393
Fierro S, Watanabe T, Akai K, Einaga Y (2012) Highly sensitive detection of Cr6+ on boron doped diamond electrodes. Electrochim Acta 82:9–11
Qi YY, Chen YT, Xiu FR, Hou JX (2020) An aptamer-based colorimetric sensing of acetamiprid in environmental samples: convenience, sensitivity and practicability. Sensors Actuators B Chem 304:127359
Chang YQ, Zhang Z, Hao JH, Yang WS, Tang JL (2016) BSA-stabilized Au clusters as peroxidase mimetic for colorimetric detection of Ag+. Sensors Actuators B Chem 232:692–697
Li ZX, Feng KZ, Zhang W, Ma M, Gu N, Zhang Y (2018) Catalytic mechanism and application of nanozymes. Chin Sci Bull 63(21):2128–2139
Wang XX, Liu GM, Qi YX, Yuan Y, Gao J, Luo XL, Yang T (2019) Embedded Au nanoparticles-based ratiometric electrochemical sensing strategy for sensitive and reliable detection of copper ions. Anal Chem 91(18):12006–12013
Gao LZ, Zhuang J, Nie L, Zhang JB, Zhang Y, Gu N, Wang TH, Feng J, Yang DL, Perrett S, Yan XY (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583
Josephy PD, Eling T, Mason RP (1982) The horseradish peroxidase-catalyzed oxidation of 3,5,3’,5’-tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J Biol Chem 257(7):3669–3675
Burns C, Spendel WU, Puckett S, Pacey GE (2006) Solution ionic strength effect on gold nanoparticle solution color transition. Talanta 69(4):873–876
Koutsoulis NP, Giokas DL, Vlessidis AG, Tsogas GZ (2010) Alkaline earth metal effect on the size and color transition of citrate-capped gold nanoparticles and analytical implications in periodate-luminol chemiluminescence. Anal Chim Acta 669(1–2):45–52
Chinnapongse SL, MacCuspie RI, Hackley VA (2011) Persistence of singly dispersed silver nanoparticles in natural freshwaters, synthetic seawater, and simulated estuarine waters. Sci Total Environ 409(12):2443–2450
Funding
This work was supported financially by the National Natural Science Foundation of China (No. 21605018) and the Natural Science Basic Research Project of Shaanxi Province of China (No. 2020JM-528 and No. 2021JZ-52).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 654 kb)
Rights and permissions
About this article
Cite this article
Qi, Y., Ma, J., Xiu, FR. et al. Determination of Cr(VI) based on the peroxidase mimetic catalytic activity of citrate-capped gold nanoparticles. Microchim Acta 188, 273 (2021). https://doi.org/10.1007/s00604-021-04942-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04942-7