Abstract
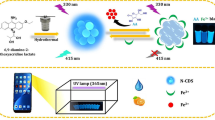
A simple dual-colour fluorescent nanoprobe has been designed composed of blue and yellow emission carbon quantum dots (CQDs). This system is inexpensive and easy to operate and was successfully employed for on-site measurements based on a smartphone app. The designed nanoprobe exhibited increased selectivity for Cr(VI), leading to a double stable response of the two CQDs. The dual-emission nanoprobe showed blue-violet fluorescence upon UV irradiation, and the fluorescent emission peaks were located at 418 nm and 552 nm. The blue light emission of CQDs was quenched with increasing Cr(VI) concentration due to the inner filter effect, whereas the yellow light emission was enhanced due to the aggregation-induced emission effect. The different responses of the dual emissions to Cr(VI) resulted in a fluorescent colour variation, thus enabling facile macroscopic visualization. With a smartphone, the change in the fluorescence colour could be observed more apparently than that of a single fluorescence nanoprobe, and the response increased linearly so that the nanoprobe could be applied to instantaneous measurements. Furthermore, the dual-emission nanoprobe was successfully employed for analysing food and water samples. Accurate concentrations were obtained by constructing a calibration plot using a fluorescence spectrometer and a smartphone app; the recoveries were 81.6% to 107.7%, and the relative standard deviation was below 3.6%. Therefore, this smartphone-integrated dual-emission detection system is promising as a new portable method for the on-site measurement of Cr(VI) ions.
Graphical abstract

* Y-CQDs: yellow emission carbon quantum dots.
B-CQDs: blue emission carbon quantum dots.
B/Y-CQDs: a mixture of B-CQDs and Y-CQDs.







Similar content being viewed by others
References
Tan X, Wang X, Hao A, Liu Y, Wang X, Chu T, Jiang L, Yang Y, Ming D (2020) Aptamer-based ratiometric fluorescent nanoprobe for specific and visual detection of zearalenone. Microchem J 157:104943. https://doi.org/10.1016/j.microc.2020.104943
Wang B, Liang Z, Tan H, Duan W, Luo M (2020) Red-emission carbon dots-quercetin systems as ratiometric fluorescent nanoprobes towards Zn2+ and adenosine triphosphate. Microchim Acta 187(6):345. https://doi.org/10.1007/s00604-020-04316-5
Tan H, Wu X, Weng Y, Lu Y, Huang Z-Z (2020) Self-assembled FRET Nanoprobe with metal-organic framework as a scaffold for Ratiometric detection of Hypochlorous acid. Anal Chem 92(4):3447–3454. https://doi.org/10.1021/acs.analchem.9b05565
Wang Y-Q, Zhao T, He X-W, Li W-Y, Zhang Y-K (2014) A novel core-satellite CdTe/silica/au NCs hybrid sphere as dual-emission ratiometric fluorescent probe for Cu2+. Biosens Bioelectron 51:40–46. https://doi.org/10.1016/j.bios.2013.07.028
Doussineau T, Schulz A, Lapresta-Fernandez A, Moro A, Körsten S, Trupp S, Mohr GJ (2010) On the Design of Fluorescent Ratiometric Nanosensors. Chem Eur J 16(34):10290–10299. https://doi.org/10.1002/chem.201000829
Li P, Xie T, Fan N, Li K, Tang B (2011) Ratiometric fluorescence imaging for distinguishing chloride concentration between normal and ischemic ventricular myocytes. Chemical communications (Cambridge, England) 48:2077-2079. https://doi.org/10.1039/c1cc15258k
Yu L, Zhang L, Ren G, Li S, Zhu B, Chai F, Qu F, Wang C, Su Z (2018) Multicolorful fluorescent-nanoprobe composed of au nanocluster and carbon dots for colorimetric and fluorescent sensing Hg2+ and Cr6+. Sensors Actuators B Chem 262:678–686. https://doi.org/10.1016/j.snb.2018.01.192
Liu W, Wang X, Wang Y, Li J, Shen D, Kang Q, Chen L (2018) Ratiometric fluorescence sensor based on dithiothreitol modified carbon dots-gold nanoclusters for the sensitive detection of mercury ions in water samples. Sensors Actuators B Chem 262:810–817. https://doi.org/10.1016/j.snb.2018.01.222
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4(4):361–377. https://doi.org/10.1016/j.arabjc.2010.07.019
Das A, Mishra S (2009) Hexavalent chromium (VI): environment pollutant and health hazard. J Environ Res Dev 2:386–392
Das R, Bandyopadhyay R, Pramanik P (2018) Carbon quantum dots from natural resource: a review. Materials Today Chemistry 8:96–109. https://doi.org/10.1016/j.mtchem.2018.03.003
Sharma P, Rajput P, Thakur A, Kim K-H, Kumar P (2019) Recent advances in carbon quantum dot-based sensing of heavy metals in water. TrAC Trends Anal Chem 114:171–195. https://doi.org/10.1016/j.trac.2019.03.003
Xu S, Zhang F, Xu L, Liu X, Ma P, Sun Y, Wang X, Song D (2018) A fluorescence resonance energy transfer biosensor based on carbon dots and gold nanoparticles for the detection of trypsin. Sensors Actuators B Chem 273:1015–1021. https://doi.org/10.1016/j.snb.2018.07.023
Zhang S, Lin B, Yu Y, Cao Y, Guo M, Shui L (2018) A ratiometric nanoprobe based on silver nanoclusters and carbon dots for the fluorescent detection of biothiols. Spectrochim Acta A Mol Biomol Spectrosc 195:230–235. https://doi.org/10.1016/j.saa.2018.01.078
Wang L, Cao H-X, He Y-S, Pan C-G, Sun T-K, Zhang X-Y, Wang C-Y, Liang G-X (2019) Facile preparation of amino-carbon dots/gold nanoclusters FRET ratiometric fluorescent probe for sensing of Pb2+/Cu2+. Sensors Actuators B Chem 282:78–84. https://doi.org/10.1016/j.snb.2018.11.058
Vaz R, Bettini J, Junior J, Lima E, Botero W, Carinhanha Caldas Santos J, Schiavon M (2017) High luminescent carbon dots as an eco-friendly fluorescence sensor for Cr(VI) determination in water and soil samples. J Photochem Photobiol A Chem 346:502–511. https://doi.org/10.1016/j.jphotochem.2017.06.047
Zhao J, Li F, Zhang S, An Y, Sun S (2019) Preparation of N-doped yellow carbon dots and N, P co-doped red carbon dots for bioimaging and photodynamic therapy of tumors. New J Chem 43(16):6332–6342. https://doi.org/10.1039/C8NJ06351F
Zhang R, Chen W (2014) Nitrogen-doped carbon quantum dots: facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens Bioelectron 55:83–90. https://doi.org/10.1016/j.bios.2013.11.074
Campos BB, Oliva MM, Contreras-Cáceres R, Rodriguez-Castellón E, Jiménez-Jiménez J, da Silva JCGE, Algarra M (2016) Carbon dots on based folic acid coated with PAMAM dendrimer as platform for Pt(IV) detection. J Colloid Interface Sci 465:165–173. https://doi.org/10.1016/j.jcis.2015.11.059
Song Y, Zhu C, Song J, Li H, Du D, Lin Y (2017) Drug-derived bright and color-tunable N-doped carbon dots for cell imaging and sensitive detection of Fe3+ in living cells. ACS Appl Mater Interfaces 9(8):7399–7405. https://doi.org/10.1021/acsami.6b13954
Song L, Cui Y, Zhang C, Hu Z, Liu X (2016) Microwave-assisted facile synthesis of yellow fluorescent carbon dots from o-phenylenediamine for cell imaging and sensitive detection of Fe3+ and H2O2. RSC Adv 6(21):17704–17712. https://doi.org/10.1039/C6RA02554D
Vedamalai M, Periasamy AP, Wang C-W, Tseng Y-T, Ho L-C, Shih C-C, Chang H-T (2014) Carbon nanodots prepared from o-phenylenediamine for sensing of Cu2+ ions in cells. Nanoscale 6(21):13119–13125. https://doi.org/10.1039/C4NR03213F
Shieh Y-T, Chen J-Y, Twu Y-K, Chen W-J (2012) The effect of pH and ionic strength on the dispersion of carbon nanotubes in poly(acrylic acid) solutions. Polym Int 61(4):554–559. https://doi.org/10.1002/pi.3203
Ju J, Zhang R, He S, Chen W (2014) Nitrogen-doped graphene quantum dots-based fluorescent probe for the sensitive turn-on detection of glutathione and its cellular imaging. RSC Adv 4(94):52583–52589. https://doi.org/10.1039/C4RA10601F
Zhang Y, Cui P, Zhang F, Feng X, Wang Y, Yang Y, Liu X (2016) Fluorescent probes for “off–on” highly sensitive detection of Hg2+ and L-cysteine based on nitrogen-doped carbon dots. Talanta 152:288–300. https://doi.org/10.1016/j.talanta.2016.02.018
Zhuo Y, Miao H, Zhong D, Zhu S, Yang X (2015) One-step synthesis of high quantum-yield and excitation-independent emission carbon dots for cell imaging. Mater Lett 139:197–200. https://doi.org/10.1016/j.matlet.2014.10.048
Kim H, Lee B-I, Byeon S-H (2015) The inner filter effect of Cr(vi) on Tb-doped layered rare earth hydroxychlorides: new fluorescent adsorbents for the simple detection of Cr(vi). Chem Commun 51(4):725–728. https://doi.org/10.1039/C4CC08543D
Zhang D, Dong Z, Jiang X, Feng M, Li W, Gao G (2013) A proof-of-concept fluorescent strategy for highly selective detection of Cr(vi) based on inner filter effect using a hydrophilic ionic chemosensor. Anal Methods 5(7):1669–1675. https://doi.org/10.1039/C3AY26555B
Sun J, Zhao J, Wang L, Li H, Yang F, Yang X (2018) Inner filter effect-based sensor for horseradish peroxidase and its application to fluorescence immunoassay. ACS Sensors 3(1):183–190. https://doi.org/10.1021/acssensors.7b00830
Gu W, Pei X, Cheng Y, Zhang C, Zhang J, Yan Y, Ding C, Xian Y (2017) Black phosphorus quantum dots as the Ratiometric fluorescence probe for trace mercury ion detection based on inner filter effect. ACS Sensors 2(4):576–582. https://doi.org/10.1021/acssensors.7b00102
Jia X, Li J, Wang E (2013) Cu Nanoclusters with aggregation induced emission enhancement. Small 9(22):3873–3879. https://doi.org/10.1002/smll.201300896
Park S, Seo J, Kim SH, Park SY (2008) Tetraphenylimidazole-based excited-state Intramolecular proton-transfer molecules for highly efficient blue electroluminescence. Adv Funct Mater 18(5):726–731. https://doi.org/10.1002/adfm.200700827
Jain CK, Bandyopadhyay A, Bhadra A (2010) Assessment of ground water quality for drinking purpose, district Nainital, Uttarakhand, India. Environ Monit Assess 166(1):663–676. https://doi.org/10.1007/s10661-009-1031-5
Chuanxi W, Jiang K, Xu Z, Lin H, Zhang C (2016) Glutathione modified carbon-dots: from aggregation-induced emission enhancement property to “turn-on” sensing of temperature/Fe3+ ions in cells. Inorg Chem Front 3:514–522. https://doi.org/10.1039/C5QI00273G
Wang H, Jiang Y, Tian C, Pan R, Dang F, Feng J, Li M, Zhang Y, Li H, Man C (2018) Determination of the transfer of lead and chromium from feed to raw milk in Holstein cows. Food Additives & Contaminants: Part A 35(10):1990–1999. https://doi.org/10.1080/19440049.2018.1496279
Organization W (2011) Guidelines for drinking-water quality
Zhang H, Wang Y, Xiao S, Wang H, Wang J-H, Feng L (2016) Rapid detection of Cr(VI) ions based on cobalt(II)-doped carbon dots. Biosens Bioelectron 87:46–52. https://doi.org/10.1016/j.bios.2016.08.010
Xu W, Yu L, Xu H, Zhang S, Xu W, Lin Y, Zhu X (2019) Water-dispersed silicon quantum dots for on-off-on fluorometric determination of chromium(VI) and ascorbic acid. Microchim Acta 186(10):673. https://doi.org/10.1007/s00604-019-3751-8
Huang S, Qiu H, Zhu F, Lu S, Xiao Q (2015) Graphene quantum dots as on-off-on fluorescent probes for chromium(VI) and ascorbic acid. Microchim Acta 182(9):1723–1731. https://doi.org/10.1007/s00604-015-1508-6
Acknowledgements
This work was supported by the Open Project Program of the State Key Laboratory of Food Nutrition and Safety, Tianjin University of Science & Technology (No. SKLFNS-KF-201908), and the National Natural Science Foundation of China (No. 21806083).
Author information
Authors and Affiliations
Contributions
Yao Chixuan: Conceptualization, Methodology, Investigation, Writing - Original Draft. Liu Qingrun: Data Curation, Validation. Zhao Ning: Visualization. Liu Jing-Min: Writing- Reviewing and Editing. Fang Guozhen: Project administration. Wang Shuo: Supervision, Funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 6219 kb)
Rights and permissions
About this article
Cite this article
Yao, C., Liu, Q., Zhao, N. et al. Ratiometric determination of Cr(VI) based on a dual-emission fluorescent nanoprobe using carbon quantum dots and a smartphone app. Microchim Acta 188, 89 (2021). https://doi.org/10.1007/s00604-021-04747-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04747-8