Abstract
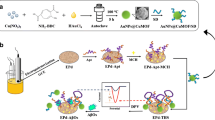
In order to overcome the antibody-based sensor’s shortcomings, an electrochemical aptamer (Apt)-based sensor was developed for amyloid-β40 oligomer (Aβ40-O). The aptasensor was constructed by locating Apt and ferrocence (Fc) on streptavidin-modified gold (SA-gold) nanoparticles. The obtained AptFc@SA-gold nanoparticles were linked onto the Au electrode via the connection of double-stranded DNA (dsDNA) as a “conductive spring.” The determination of Aβ40-O was performed with square-wave voltammetry (SWV). Upon bio-recognition between Apt and Aβ40-O, the conformation of Apt changed and the formed Apt/Aβ40-O complex separated from the SA-gold surface. As a result, the surface charge of SA-gold positively shifted, weakening the electrostatic attraction between the SA-gold and the positively charged Au electrode surface (at potential range of 0.1~0.5 V, corresponding to the Fc redox transformation), and stretching the dsDNA chain. Based on the exponential decay of dsDNA’s electron transfer efficiency on its chain stretching, the oxidation current density from Fc decreased and displayed linear correlation to the concentration of Aβ40-O. A wide linear range of 0.100 nM to 1.00 μM with a low detection limit of 93.0 pM was obtained. The aptasensor displayed excellent selectivity toward Aβ40-O in contrast to other possible interfering analogs (Aβ40 monomer, Aβ42 monomer, and oligomer) at × 100 higher concentrations. The recoveries for Aβ40-O-spiked artificial cerebrospinal fluid and healthy human serum were 94.0~104% and 92.8~95.4%, respectively. The electrochemical aptasensor meets the demands of clinic determination of Aβ40-O, which is significant for the early diagnosis of AD.

Schematic representation of the electrochemical aptasensor for amyloid-β oligomer based on the surface charge change induced by target binding.




Similar content being viewed by others
References
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s Dement 9:63–75
Mroczko B, Groblewska M, Litman-Zawadzka A, Kornhuber J, Lewczuk P (2018) Amyloid β oligomers (AβOs) in Alzheimer’s disease. J Neural Transm 125:177–191
Lewczuk P, Kornhuber J (2011) Neurochemical dementia diagnostics in Alzheimer’s disease: where are we now and where are we going? Expert Rev Proteomics 8:447–458
Wang J, Zhuo Y, Zhou Y, Wang H, Yuan R, Chai Y (2016) Ceria doped zinc oxide nanoflowers enhanced luminol-based electrochemiluminescence immunosensor for amyloid-β detection. ACS Appl Mater Interfaces 8:12968–12975
Qu F, Yang M, Rasooly A (2016) Dual signal amplification electrochemical biosensor for monitoring the activity and inhibition of the Alzheimer’s related protease β-secretase. Anal Chem 88:10559–10565
Xia N, Wang X, Zhou B, Wu Y, Mao W, Liu L (2016) Electrochemical detection of amyloid-β oligomers based on the signal amplification of a network of silver nanoparticles. ACS Appl Mater Interfaces 8:19303–19311
Negahdary M, Heli H (2019) An electrochemical peptide-based biosensor for the Alzheimer biomarker amyloid-β(1-42) using a microporous gold nanostructure. Microchim Acta 186:766–772
Thies B, Truschke E, Morrison-Bogorad M, Hodes RJ (1999) Consensus report of the Working Group on: molecular and biochemical markers of Alzheimer’s disease. Neurobiol Aging 20:109–116
Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, Deyn PPD, Bancher C, Cras P, Wiltfang J, Mehta PD, Iqbal K, Pottel H, Vanmechelen E, Vanderstichele H (1999) Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology 52:1555–1562
Xia N, Liu L, Harrington MG, Wang J, Zhou F (2010) Regenerable and simultaneous surface plasmon resonance detection of aβ(1-40) and aβ(1-42) peptides in cerebrospinal fluids with signal amplification by streptavidin conjugated to an N-terminus-specific antibody. Anal Chem 82:10151–10157
Golde TE, Eckman CB, Younkin SG (2000) Biochemical detection of Abeta isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. Biochim Biophys Acta 1502:172–187
Munishkina LA, Fink AL (2007) Fluorescence as a method to reveal structures and membrane-interactions of amyloidogenic proteins. Biochim Biophys Acta 1768:1862–1885
Deng C, Liu H, Zhang M, Deng H, Lei C, Shen L, Jiao B, Tu Q, Jin Y, Xiang L, Deng W, Xie Y, Xiang J (2018) Light-up nonthiolated aptasensor for low-mass, soluble amyloid-beta40 oligomers at high salt concentrations. Anal Chem 90:1710–1717
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Robertson DL, Joyce GF (1990) Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 344:467–468
Deng C, Chen J, Nie L, Nie Z, Yao S (2009) Sensitive bifunctional aptamer-based electrochemical biosensor for small molecules and protein. Anal Chem 81:9972–9978
Ren K, Wu J, Yan F, Ju H (2014) Ratiometric electrochemical proximity assay for sensitive one-step protein detection. Sci Rep 4:4360
Wilson MS, Nie W (2006) Multiplex measurement of seven tumor markers using an electrochemical protein chip. Anal Chem 78:6476–6483
Voityuk AA (2009) Can charge transfer in DNA significantly be modulated by varying the pi stack conformation? J Phys Chem B 113:14365–14368
Bruot C, Xiang L, Palma J, Tao N (2015) Effect of mechanical stretching on DNA conductance. ACS Nano 9:88–94
Zhou Y, Huang Z, Yang R, Liu J (2017) Selection and screening of DNA aptamers for inorganic nanomaterials. Chemistry 24:2525–2532
Tsukakoshi K, Abe K, Sode K, Ikebukuro K (2012) Selection of DNA aptamers that recognize α-synuclein oligomers using a competitive screening method. Anal Chem 84:5542–5547
Deng C, Zhang M, Liu C, Deng H, Huang Y, Yang M, Xiang J, Ren B (2018) Electrostatic force triggering elastic condensation of double-stranded DNA for high-performance one-step immunoassay. Anal Chem 90:11446–114521
Deng C, Liu H, Zhang M, Deng H, Lei C, Shen L, Jiao B, Tu Q, Jin Y, Xiang L, Deng W, Xie Y, Xiang J (2018) Light-up nonthiolated aptasensor for low-mass, soluble amyloid Aβ40 oligomers at high salt concentrations. Anal Chem 90:1710–1717
Zhang M, Chen J, Pi X, Deng C, Xiang J (2019) Construction and electrochemical property studies of DNA duplexes tethered to gold electrode via Au-C bond. Electroanal 3:477–484
Fu Y, Chen S, Kuzume A, Rudnev A, Huang C, Kaliginedi V, Baghernejad M, Hong W, Wandlowski T, Decurtins S, Liu SX (2015) Exploitation of desilylation chemistry in tailor-made functionalization on diverse surfaces. Nat Commun 6:6403–6409
Wang K (2018) DNA-based single-molecule electronics: from concept to function. J Funct Biomater 9:1–31
Shen W, Zhuo Y, Chai Y, Yuan R (2015) Cu-based metal-organic frameworks as a catalyst to construct a ratiometric electrochemical aptasensor for sensitive lipopolysaccharide detection. Anal Chem 87:11345–11352
Xu D, Xu D, Yu X, Liu Z, He W, Ma Z (2005) Label-free electrochemical detection for aptamer-based array electrodes. Anal Chem 77:5107–5113
Veloso A, Chow A, Ganesh H, Li N, Dhar D, Wu D, Mikhaylichenko S, Brown I, Kerman K (2014) Electrochemical immunosensors for effective evaluation of amyloid-beta modulators on oligomeric and fibrillar aggregation processes. Anal Chem 86:4901–4909
Du Y, Lim BJ, Li B, Jiang YS, Sessler JL, Ellington AD (2014) Reagentless, ratiometric electrochemical DNA sensors with improved robustness and reproducibility. Anal Chem 86:8010–8016
She W, Luo K, Zhang C, Wang G, Geng Y, Li L, He B, Gu Z (2013) The potential of self-assembled, pH-responsive nanoparticles of mPEGylated peptide dendron-doxorubicin conjugates for cancer therapy. Biomaterials 34:1613–1623
Guerrini L, Arenal R, Mannini B, Chiti F, Pini R, Matteini P, Alvarezpuebla R (2015) SERS detection of amyloid oligomers on metallorganic-decorated plasmonic beads. ACS Appl Mater Interfaces 7:9420–9428
Kim J, Kim M, Kang S, Lim K, Kim T, Ji Y (2015) Magnetic bead droplet immunoassay of oligomer amyloid β for the diagnosis of Alzheimer’s disease using micro-pillars to enhance the stability of the oil–water interface. Biosens Bioelectron 67:724–732
Pihlasalo S, Deguchi T, Virtamo M, Jacobino J, Chary K, López-Picón FR, Brunhofer-Bolzer G, Huttunen R, Fallarero A, Vuorela P (2017) Luminometric nanoparticle-based assay for high sensitivity detection of β-amyloid aggregation. Anal Chem 89:2398–2404
Veloso A, Hung V, Sindhu G, Constantinof A, Kerman K (2009) Electrochemical oxidation of benzothiazole dyes for monitoring amyloid formation related to the Alzheimer’s disease. Anal Chem 81:9410–9415
Funding
This work was financially supported by the National High Technology Research and Development Program of China (2015AA020502), the National Key Basic Research Program of China (2014CB744502), the National Natural Science Foundation of China (21727810, 21573290, 21005090), and Hunan Provincial Natural Science Foundation of China (13JJ3004), Hunan Provincial Science and Technology Plan Project, China (2016TP1007), and the China Postdoctoral Science Foundation (2012M510136, 2013T60774).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 134 kb)
Rights and permissions
About this article
Cite this article
Deng, C., Liu, H., Si, S. et al. An electrochemical aptasensor for amyloid-β oligomer based on double-stranded DNA as “conductive spring”. Microchim Acta 187, 239 (2020). https://doi.org/10.1007/s00604-020-4217-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4217-8