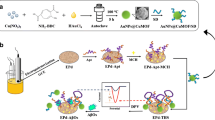
Abstract
An excellent aptasensor for electrochemical detection of amyloid-β oligomers (AβOs) at trace levels was fabricated based on a triple-helix aptamer switch (THAS) via target-triggered signal transduction DNA displacement events. Specifically, a single-stranded anti-AβO aptamer (Apt) carrying two symmetrical arm segments was first attached via Au-S binding to an Au electrode. Gold nanoparticle (GNP)-tagged signal transduction probes (GNP-STPs) were simultaneously hybridized with the two arm segments of the Apt, and a rigid THAS was formed on the Au electrode. Compared to the conventional hybrid, the number of GNPs on the Au electrode increased significantly with the THAS, effectively improving the stability of the Apt to avoid lodging. Trithiocyanuric acid (TA) was utilized to further gather the GNPs and form network-like TA/GNPs. As a result, the differential pulse voltammetry (DPV) response of GNPs was clearly enhanced. When AβOs were present, target-triggered signal transduction DNA displacement events were carried out from THAS via the reaction of the Apt with the AβOs, which caused the GNP-STP to dissociate from the Au electrode, and thus a significant reduction in the DPV response was observed. The assay was able to sensitively detect trace AβOs by monitoring the AβO-controlled DPV response change. It exhibited a wide linear range from 1 fM to 10 pM with a low detection limit of 0.5 fM, and was successfully employed for the determination of AβOs in 20 serum samples, with good recovery. Moreover, the developed assay can provide a sensitive and selective platform for many studies or investigations related to Alzheimer’s disease (AD) monitoring and treatment.
Graphical abstract







Similar content being viewed by others
References
Kaya I, Zetterberg H, Blennow K, Hanrieder J. Shedding light on the molecular pathology of amyloid plaques in transgenic Alzheimer’s disease mice using multimodal MALDI imaging mass spectrometry. ACS Chem Neurosci. 2018;9:1802–17.
Ryan P, Patel B, Makwana V, Jadhav HR, Kiefel M, Davey A, et al. Peptides, peptidomimetics, and carbohydrate-peptide conjugates as amyloidogenic aggregation inhibitors for Alzheimer’s disease. ACS Chem Neurosci. 2018;9:1530–51.
Bieschke J, Herbst M, Wiglenda T, Friedrich RP, Boeddrich A, Schiele F, et al. Small-molecule conversion of toxic oligomers to nontoxic beta-sheet-rich amyloid fibrils. Nat Chem Biol. 2012;8:93–101.
Hamley IW. The amyloid beta peptide: a chemist’s perspective role in Alzheimer’s and fibrillization. Chem Rev. 2012;112:5147–92.
Rabbito A, Dulewicz M, Kulczynska-Przybik A, Mroczko B. Biochemical markers in Alzheimer’s disease. Int J Mol Sci. 2020;21:1989.
Jamerlan A, An SSA, Hulme J. Advances in amyloid beta oligomer detection applications in Alzheimer’s disease. TrAC Trends Anal Chem. 2020;129:115919.
Zhang XL, Tian YL, Zhang C, Tian XY, Ross AW, Moir RD, et al. Near-infrared fluorescence molecular imaging of amyloid beta species and monitoring therapy in animal models of Alzheimer’s disease. Proc Natl Acad Sci. 2015;112:9734–9.
Raymond SB, Treat LH, Dewey JD, McDannold NJ, Hynynen K, Bacskai BJ. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer’s disease mouse models. PLoS One. 2008;3:e2175.
Smith SV. Molecular imaging with copper-64. J Inorg Biochem. 2004;98:1874–901.
Bruggink KA, Jongbloed W, Biemans EALM, Veerhuis R, Claassen JAHR, Kuiperij HB, et al. Amyloid-β oligomer detection by ELISA in cerebrospinal fluid and brain tissue. 2013. Anal Biochem. 2013;433:112–20.
Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101.
Cernescu M, Stark T, Kalden E, Kurz C, Leuner K, Deller T, et al. Laser-induced liquid bead ion desorption mass spectrometry: an approach to precisely monitor the oligomerization of the β-amyloid peptide. Anal Chem. 2012;8:5276–84.
Jin XF, Liu CH, Xu TL, Su L, Zhang XJ. Artificial intelligence biosensors: challenges and prospects. Biosens Bioelectron. 2020;165:112412.
Veloso AJ, Chow AM, Ganesh HVS, Li N, Dhar D, Wu DCH, et al. Electrochemical immunosensors for effective evaluation of amyloid-beta modulators on oligomeric and fibrillare aggregation processes. Anal Chem. 2014;86:4901–9.
Sun L, Zhong Y, Gui J, Wang X, Zhuang X, Weng J. A hydrogel biosensor for high selective and sensitive detection of amyloid-beta oligomers. Int J Nanomedicine. 2018;13:843–56.
Zhu LL, Zhang JY, Wang FY, Wang Y, Lu LL, Feng CC, et al. Selective amyloid β oligomer assay based on abasic site-containing molecular beacon and enzyme-free amplification. Biosens Bioelectron. 2016;78:206–12.
Qin J, Jo DG, Cho M, Lee Y. Monitoring of early diagnosis of Alzheimer’s disease using the cellular prion protein and poly(pyrrole-2-carboxylic acid) modified electrode. Biosens Bioelectron. 2018;113:82–7.
Li H, Xie HN, Cao Y, Ding XR, Yin YM, Li GX. A general way to assay protein by coupling peptide with signal reporter via supermolecule formation. Anal Chem. 2013;85:1047–52.
Zhou J, Meng LC, Ye WR, Wang QL, Geng SZ, Sun C. A sensitive detection assay based on signal amplification technology for Alzheimer’s disease’s early biomarker in exosome. Anal Chim Acta. 2018;1022:124–30.
Deng CY, Liu H, Zhang MM, Deng HH, Lei CY, Shen L, et al. Light-up nonthiolated aptasensor for low-mass, soluble amyloid-β 40 oligomers at high salt concentrations. Anal Chem. 2018;90:1710–7.
Zhou YL, Zhang HQ, Liu LT, Li CM, Chang Z, Zhu X, et al. Fabrication of an antibody-aptamer sandwich assay for electrochemical evaluation of levels of β-amyloid oligomers. Sci Rep. 2016;6:35186.
Ke H, Sha HF, Wang YF, Guo WW, Zhang X, Wang ZM, et al. Electrochemiluminescence resonance energy transfer system between GNRs and Ru(bpy)32+: application in magnetic aptasensor for β-amyloid. Biosens Bioelectron. 2018;100:266–73.
Zhang YT, Figueroa-Miranda G, Lyu ZZ, Zafiu C, Willbold D, Offenhausser AD, et al. Monitoring amyloid-beta proteins aggregation based on label-free aptasensor. Sensor Actuat B-Chem. 2019;288:535–42.
Zhou Y, Wang HJ, Zhuo Y, Chai YQ, Yuan R. Highly efficient electrochemiluminescent silver nanoclusters/titanium oxide nanomaterials as a signal probe for ferrocene-driven light switch bioanalysis. Anal Chem. 2017;89:3732–8.
Wang YF, Zhang Y, Sha HF, Xiong X, Jia NQ. Design and biosensing of a ratiometric electrochemiluminescence resonance energy transfer aptasensor between a g-C3N4 Nanosheet and Ru@MOF for amyloid-β protein. ACS Appl Mater Interfaces. 2019;11:36299–306.
You M, Yang S, An Y, Zhang F, He PG. A novel electrochemical biosensor with molecularly imprinted polymers and aptamer-based sandwich assay for determining amyloid-β oligomer. J Electroanal Chem. 2020;862:114017.
Nie GM, Bai ZM, Yu WY, Chen J. Electrochemiluminescence biosensor based on conducting poly(5-formylindole) for sensitive detection of ramos cells. Biomacromolecules. 2013;14:834–40.
Bagheri E, Abnous K, Alibolandi M, Ramezani M, Taghdisi SM. Triple-helix molecular switch-based aptasensors and DNA sensors. Biosens Bioelectron. 2018;111:1–9.
Xiao Y, Piorek BD, Plaxco KW, Heeger AJ. A reagentless signal-on architecture for electronic, aptamer-based sensors via target-induced strand displacement. J Am Chem Soc. 2005;127:17990–1.
Shamsipur M, Farzin L, Tabrizi MA. Ultrasensitive aptamer-based on-off assay for lysozyme using a glassy carbon electrode modified with gold nanoparticles and electrochemically reduced graphene oxide. Microchim Acta. 2016;183:2733–43.
Zhang ZP, Tao CC, Yin JG, Wang YH, Li YS. Enhancing the response rate of strand displacement-based electrochemical aptamer sensors using bivalent binding aptamer-cDNA probes. Biosens Bioelectron. 2018;103:39–44.
Doluca O, Withers JM, Filichev VV. Molecular engineering of guanine-rich sequences: Z-DNA, DNA triplexes, and G-quadruplexes. Chem Rev. 2013;113:3044–83.
Wang XZ, Jiang AW, Hou T, Li F. A versatile label-free and signal-on electrochemical biosensing platform based on triplex-forming oligonucleotide probe. Anal Chim Acta. 2015;890:91–7.
Abnous K, Danesh NM, Ramezani M, Alibolandi M, Hassanabad KY, Emrani AS, et al. A triple-helix molecular switch-based electrochemical aptasensor for interferon-gamma using a gold electrode and methylene blue as a redox probe. Microchim Acta. 2017;184:4151–7.
Liu QQ, Liu JQ, He DG, Qing TP, He XX, Wang KM, et al. Triple-helix molecular switch-induced hybridization chain reaction amplification for developing a universal and sensitive electrochemical aptasensor. RSC Adv. 2016;6:90310–7.
Mao YF, Liu JQ, He DG, He XX, Wang KM, Shi H, et al. Aptamer/target binding-induced triple helix forming for signal-on electrochemical biosensing. Talanta. 2015;143:381–7.
Stine WB, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–22.
Saha K, Agasti SS, Kim C, Li XN, Rotello VM. Gold nanoparticles in chemical and biological sensing. Chem Rev. 2012;112:2739–79.
Song YJ, Feng LY, Ren JS, Qu XG. Stabilization of unstable CGC(+) triplex DNA by single-walled carbon nanotubes under physiological conditions. Nucleic Acids Res. 2011;39:6835–43.
Huang CC, Huang YF, Cao ZH, Tan WH, Chang HT. Aptamer-modified gold nanoparticles for colorimetric determination of platelet-derived growth factors and their receptors. Anal Chem. 2005;77:5735–41.
Funding
The work was supported by the Qinglan Project funded by universities in Jiangsu and the Fundamental Research Funds for the Central Universities (No.2242020 K40191).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The collections and experiments on the human biological samples were approved by the Ethics Committee of Zhongda Hospital affiliated with Southeast University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, X., Gu, X., Li, L. et al. An excellent electrochemical aptasensor for amyloid-β oligomers based on a triple-helix aptamer switch via target-triggered signal transduction DNA displacement events. Anal Bioanal Chem 413, 3707–3716 (2021). https://doi.org/10.1007/s00216-021-03319-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03319-2